Controversy Kicks Up Over A Drug Meant To Prevent Premature Birth
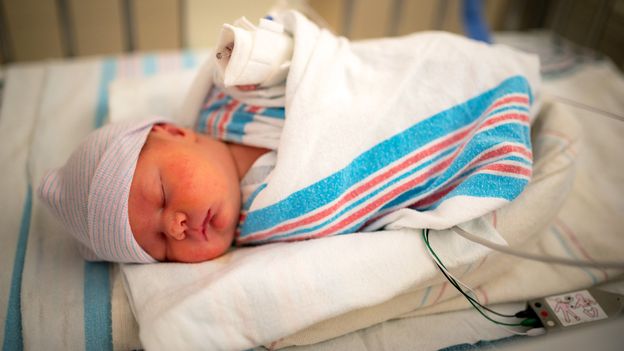
NPRControversy Kicks Up Over A Drug Meant To Prevent Premature Birth Enlarge this image toggle caption Jill Lehmann Photography/Getty Images Jill Lehmann Photography/Getty Images An independent panel of advisers to the Food and Drug Administration recommended last week that a medication to prevent preterm birth be taken off the market because, the advisers decided, the preponderance of evidence suggests it doesn't work. "There was a 66% drop in the rate of recurrent preterm birth among women taking progesterone," says Dr. Eva Pressman, chair of obstetrics and gynecology at the University of Rochester. In part, ACOG's guidance says, "A woman with a singleton gestation and a prior spontaneous preterm singleton birth should be offered progesterone supplementation starting at 16-24 weeks of gestation to reduce the risk of recurrent spontaneous preterm birth." And she notes that the University of Rochester Medical Center, where she works, will not advise discontinuing the medication for women who have already been receiving weekly injections — "because there is some data indicating that withdrawing progesterone and stopping the treatment can increase the risk of preterm birth more than if they hadn't started in the first place."
History of this topic

First Over-The-Counter Birth Control Pill Gets FDA Approval
Huff Post
Abortion clinics reassure worried patients, set backup plans
The Independent
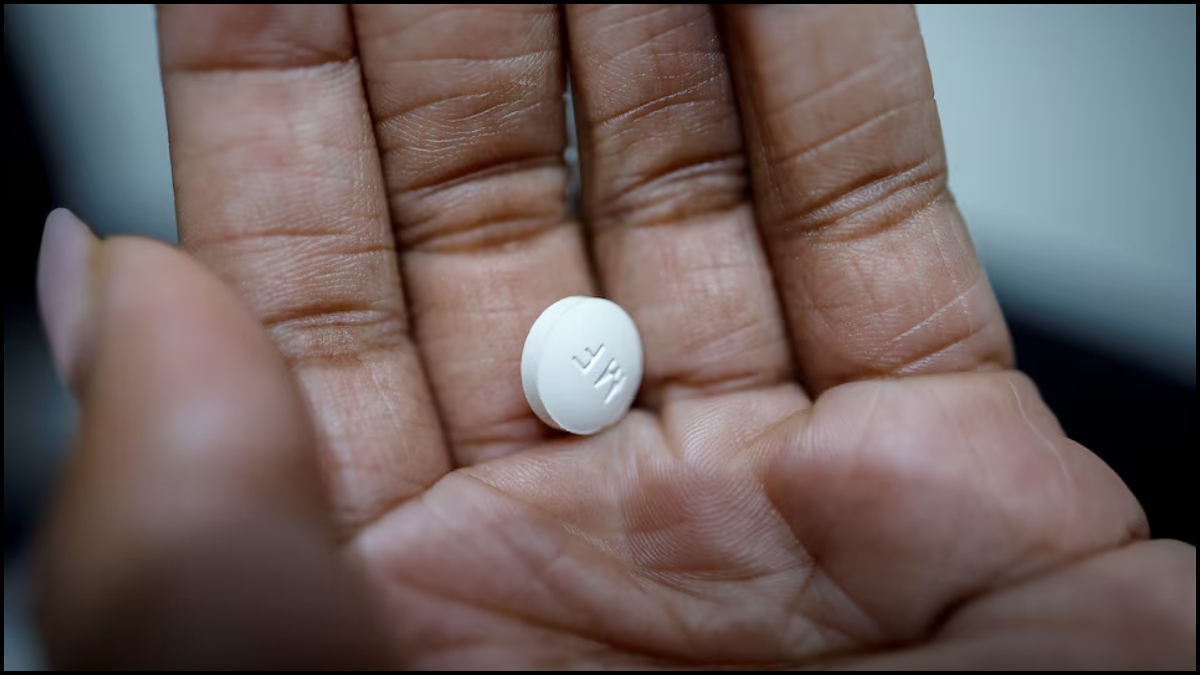
FDA pulls only drug for preterm births, saying it has no benefit
LA Times
FDA pushes to remove pregnancy drug, company pushes back
Associated Press
Science hasn’t shown these medications work. They’re being sold anyway
LA Times
Editorial: FDA should pull ineffective pregnancy drug Makena until we know it works
LA Times
Covis Pharma’s statement to The Times about Makena
LA TimesDiscover Related