Merck’s at-home antiviral COVID-19 pill approved by US regulator
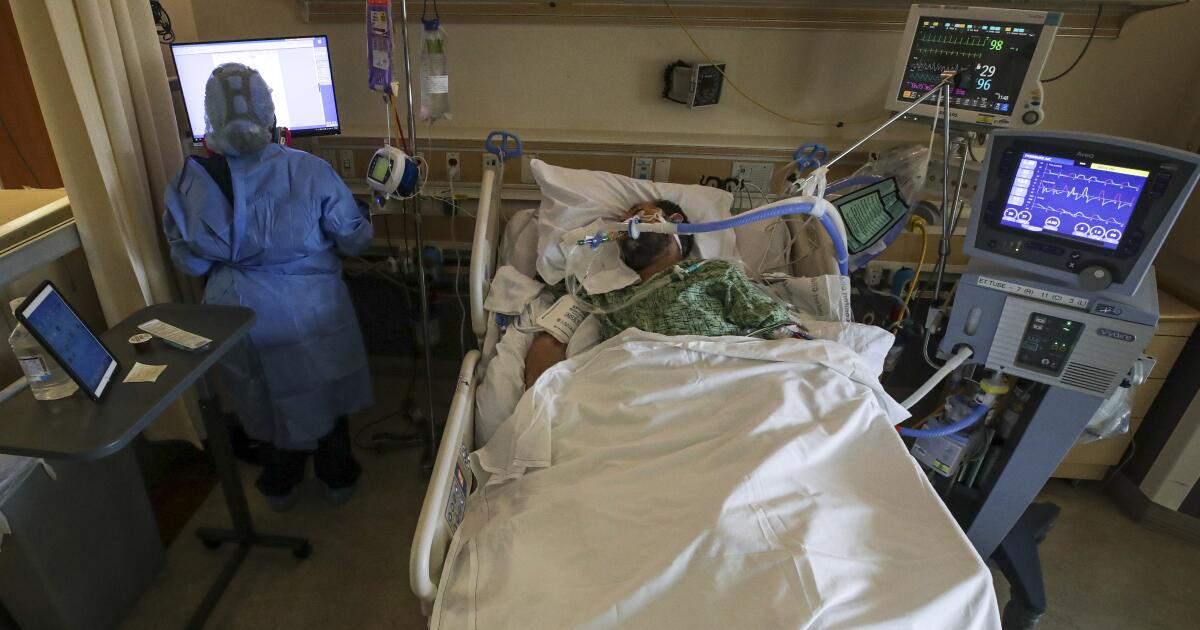
Al JazeeraUnited States health regulators have authorised a second antiviral pill against COVID-19, providing another easy-to-use medication to battle the rising tide of Omicron infections. Additionally, Pfizer’s drug was roughly three times more effective in testing than Merck’s, reducing hospitalisation and death by nearly 90 percent among high-risk patients. Merck’s drug is not authorised for use in patients younger than 18 because molnupiravir may affect bone and cartilage growth, the FDA said. “To the extent that there’s an ample supply of Pfizer’s pill, I think it won’t be used,” said Dr Gregory Poland of the Mayo Clinic, referring to the Merck drug. “There would be no reason, given it has less efficacy and a higher risk of side effects.” Dr Nick Kartsonis, Merck’s senior vice president of clinical research, said the company’s scientists are still studying the drug and they hope to eventually get it approved for use in children.
History of this topic
COVID pill Paxlovid gets full FDA approval after more than a year of emergency use
Associated Press
European committee: Regulators should deny Merck’s Lagevrio
Associated Press
Frustration is rising over Covid drug shortages in China, and there are no easy answers
CNN
Pfizer says its COVID pill doesn’t work for healthier patients
LA Times
COVID-19 pills could be a game-changer, but doctors trying to use them face hurdles
LA Times
Covid-19 pill competing with Pfizer’s looks for quick approval in Japan
Live Mint
Generic Drugmakers to Make Cheap Versions of Merck's Covid-19 Pill Molnupiravir for Poorer Nations
News 18
Cheap version of Merck Covid pill to be made for poorer nations
India Today
New Covid-19 pills carry risks: What patients should know
Live Mint
New easy-to-use Covid-19 pills come with a catch. Here's how
India TV News
Live updates: Japan approves use of Merck's COVID-19 pill
The Independent
US adds Merck pill as 2nd easy-to-use drug against COVID-19
Associated Press
US adds Merck pill as 2nd easy-to-use drug against Covid-19
India Today
U.S. adds Merck pill as 2nd easy-to-use drug against COVID-19
LA Times
France scraps order for Merck's Covid antiviral drug after disappointing trial results
India Today
Pfizer Pill Becomes First US-authorized Home Covid-19 Treatment
News 18
Pfizer pill becomes 1st U.S.-authorized home COVID treatment
LA Times
Merck's Covid-19 pill Molnupiravir found to reduce hospitalisation, death risks
India Today
Pfizer confirms COVID pill’s results, potency versus omicron
Associated PressPfizer confirms COVID pill’s results, potency versus Omicron
The Hindu
Pfizer says its COVID pill near 90% effective in clinical trials
Al Jazeera
Pfizer confirms Covid pill's potency against Omicron variant
India Today
Pfizer and Merck covid-19 pills are coming soon in the US, but other countries will have to wait
Live Mint
UK planning to rollout Merck's anti-Covid pill as soon as Christmas: Report
Hindustan Times
Merck’s covid-19 pill backed by FDA advisers
Live Mint
US panel backs first-of-a-kind COVID-19 pill from Merck
Associated Press
US panel backs Merck's first-of-a-kind Covid-19 pill
Hindustan Times
US health panel endorses Merck’s COVID-19 pill
Al Jazeera
Merck’s covid-19 pill to be reviewed by FDA advisers
Live Mint
FDA: Merck COVID pill effective, experts will review safety
Associated Press
Merck’s COVID pill shows lower efficacy in updated study
Al Jazeera
Merck asks EU drug regulator to authorise its Covid-19 pill
Hindustan Times
EU regulator recommends Merck’s COVID-19 pill for emergency use
Al Jazeera
Pfizer signs deal with UN-backed group to let other companies make its COVID-19 pill
Daily Mail
Pfizer says COVID-19 pill cut hospital, death risk by 90%
Associated Press
Pfizer says Covid-19 pill is 89% effective in preliminary assessment
Live Mint
Pfizer says its COVID-19 pill cuts hospitalisations, death by 89%
Al Jazeera
Britain becomes first country to approve Merck's Covid-19 pill molnupiravir
India TodayUK approves Merck's antiviral COVID pill molnupiravir in world first
ABC
UK authorizes Merck antiviral pill, 1st shown to treat COVID
Associated Press
UK becomes first country to approve Merck's anti-Covid pill molnupiravir
Hindustan Times
Molnupiravir: UK authorizes Merck/Ridgeback Biotherapeutics’ antiviral to treat mild-to-moderate Covid-19
CNN
Britain becomes the first nation to authorize Merck’s COVID-19 pill
LA Times
UK approves Merck’s antiviral COVID-19 pill in world first move
Al Jazeera
Merck strikes deal for global access to COVID-19 medication
Al Jazeera
EU medicines watchdog starts review of Merck COVID pill
Al Jazeera
WHO-led program aims to buy antiviral Covid-19 pills for $10
CNN
Merck Covid pill: Asian countries are rushing to buy molnupiravir but poor countries could miss out
CNN
Merck seeks first US authorization for Covid-19 tablet
Live MintDiscover Related