U.S. Health Officials Continue Pause Of Johnson & Johnson Vaccine
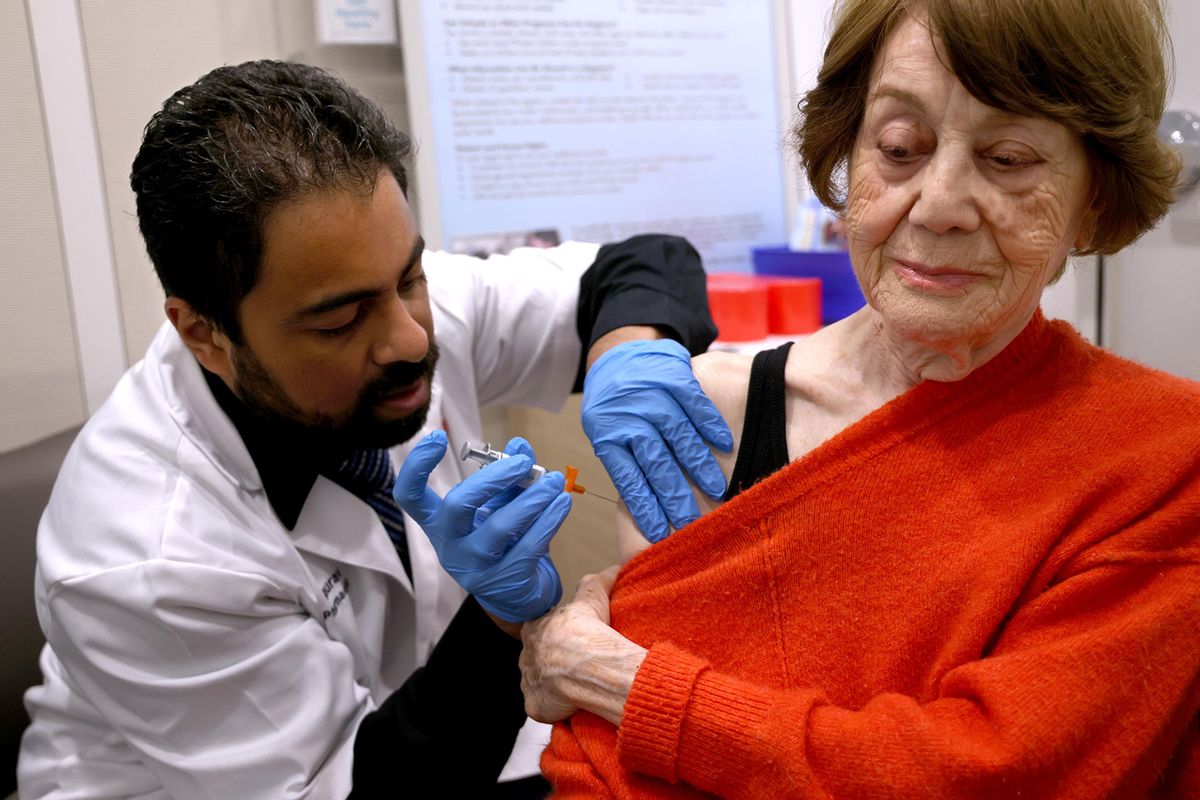
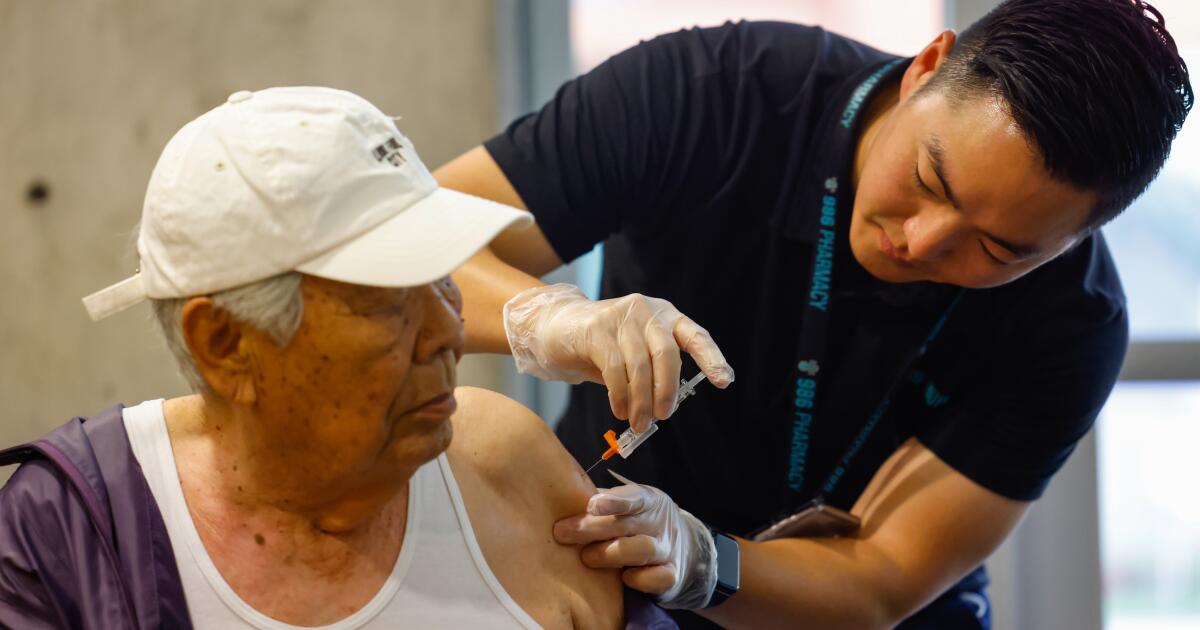
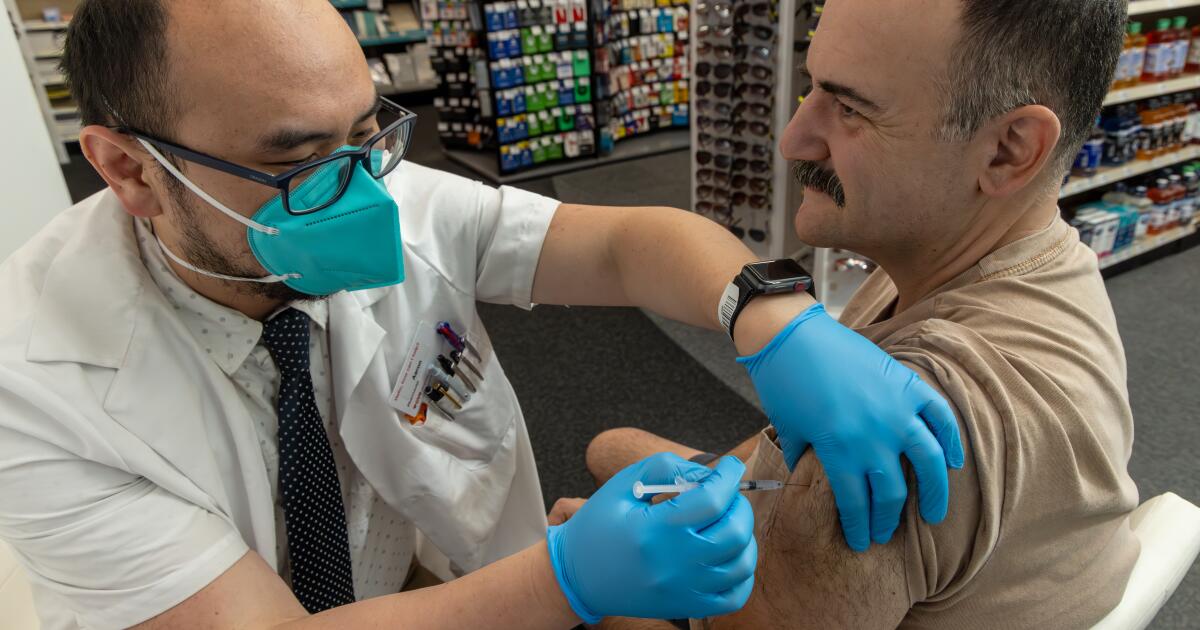
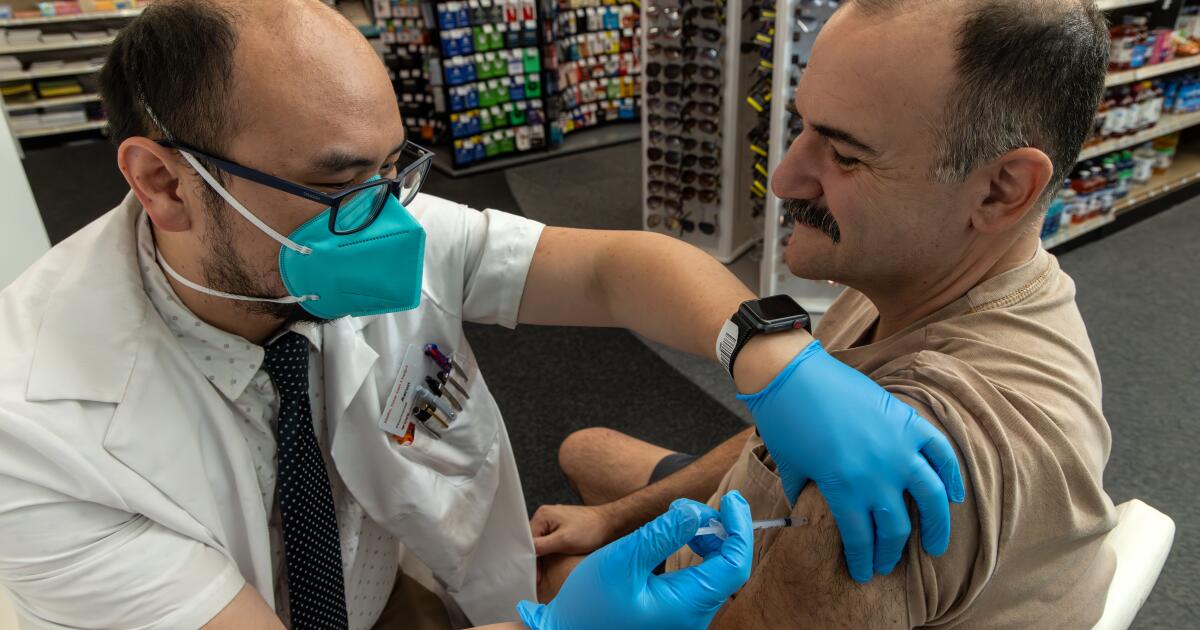
NPRU.S. Health Officials Continue Pause Of Johnson & Johnson Vaccine Enlarge this image toggle caption Frederic J. Brown/AFP via Getty Images Frederic J. Brown/AFP via Getty Images An expert advisory committee to the Centers for Disease Control and Prevention decided Wednesday it needed more time to consider whether to recommend to resume administering the COVID-19 vaccine made by Johnson & Johnson. The committee's emergency meeting took place less than 36 hours after the CDC and the Food and Drug Administration called for a voluntary pause after receiving reports of serious side effects seen in six women who had received the Johnson & Johnson vaccine. Each developed a severe blood clotting disorder called cerebral venous sinus thrombosis within 6 to 13 days after receiving the vaccine. Sponsor Message CDC director Rochelle Walensky said that officials are working to reschedule vaccine appointments for people were going to receive the Johnson & Johnson vaccine during the pause.
History of this topic
Booking a COVID-19 vaccine? Some are reporting canceled appointments or insurance issues
Associated PressAfrica's COVID vaccine production line in jeopardy
The Hindu
J&J Covid-19 vaccine ‘limited’ to some people after clot concerns: Report
Hindustan Times
FDA restricts Johnson & Johnson’s COVID-19 vaccine due to blood clot risk
LA Times
New COVID studies show promise for the Johnson & Johnson vaccine booster
NPR
US expands Covid-19 booster eligibility to all adults
India Today
New Jersey hospital becomes first to mandate BOOSTER shots for workers with J&J single dose vaccine
Daily Mail
CDC supports expansion of COVID-19 booster rollout and mixing shots
LA TimesU.S. FDA authorises 'mix and match' COVID-19 vaccine; backs Moderna, J&J boosters
The Hindu
Month On, Johnson & Johnson’s Covid Vaccines Stuck at Kasauli Lab Over Pending Data from Indian Maker
News 18
J&J vaccine recipients should get their second dose as soon as it’s available, experts say
CNN
CDC vaccine advisers schedule meetings to discuss booster shots, vaccines for kids
CNN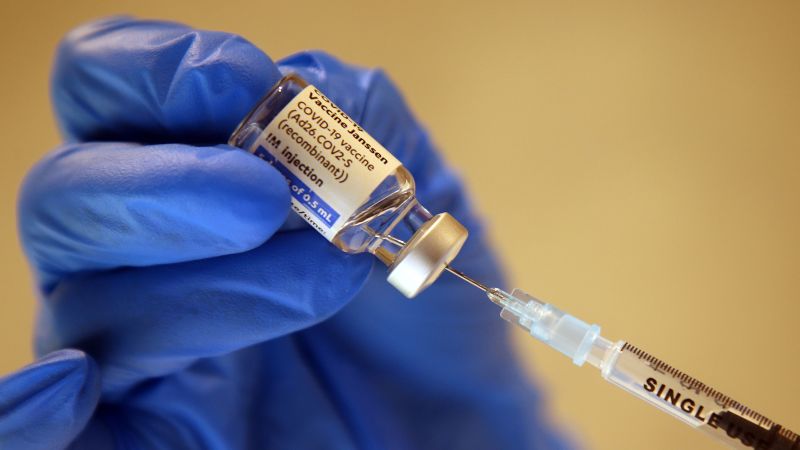
Two dose version of Johnson & Johnson shot 94% effective against Covid-19, study finds
CNN
Vaccine booster shot holdup: Some may miss the Sept. 20 start
LA Times)
Johnson & Johnson files application to test its COVID vaccine on adolescents; all you need to know
Firstpost
US health officials call for booster shots against COVID-19
Associated Press
US calls for booster shots against Covid Delta variant
India TV News
US health officials call for booster shots against COVID-19
Al Jazeera
CDC recommends extra Covid-19 vaccine dose for certain immunocompromised people
CNN
Wary of ‘breakthrough’ infections, some vaccinated people eye another shot. Is it needed?
LA Times
Johnson & Johnson Withdraws Proposal for Speedy Covid Vaccine Approval in India
News 18
Johnson & Johnson\'s single-dose vaccine shows promising signs against Delta variant
Deccan Chronicle
Johnson & Johnson's COVID Vaccine Is Effective Against The Delta Variant, Studies Find
NPR
Nearly 25% of American adults under 40 do not plan to get the COVID-19 vaccine
Daily Mail
Johnson & Johnson's vaccine drive stalls out in U.S after safety pause
India Today
Alarm in Africa: Virus surges, vaccines grind to ‘near halt’
Associated Press
9.35 million Covid vaccine doses to reach Netherlands by end-June despite Janssen delay
NL Times
Opinion: I’m 26 and I have some news for my peers on Covid-19 vaccination
CNN
100 million fully vaccinated people are helping the US reopen. But many millions more are needed
CNN
Johnson & Johnson vaccine shipments to California resume as COVID cases keep falling
LA Times
South Africa resumes giving J&J jabs to health care workers
Associated Press
CDC gives new guidance on what vaccinated people can safely enjoy. But we may be hitting a wall with vaccine hesitancy
CNN
April 26, 2021 coronavirus news
CNN
California’s COVID-19 vaccination pace slowed after Johnson & Johnson pause, data show
LA Times
US Coronavirus: CDC data shows more Americans are missing their second dose of Covid-19 vaccines
CNN)
US to resume Johnson & Johnson COVID-19 vaccinations despite rare risk of blood clots
Firstpost
US to resume J&J COVID vaccinations despite rare clot risk
Associated Press
Johnson & Johnson's COVID-19 Vaccine Is Back In Use In The U.S.
NPR
Vaccine hesitancy among Republicans emerges as Biden’s next big challenge
CNN
April 23, 2021, Covid-19 vaccine and world news
CNN
April 23, 2021, Covid-19 vaccine and world news
CNN
U.S. lifts pause on J&J vaccinations, clearing the way for shots to resume
LA Times
US has more than 9 million Johnson & Johnson doses ready to go now that pause is lifted
CNN
CDC recommends that pregnant people get a Covid-19 vaccine
CNN)
Covid Vaccine Scene in High vs Low-Income Countries: New Study Shows US Hoarding Doses
News 18
CDC vaccine advisers will meet Friday to discuss the J&J vaccine. Here’s what could happen next
CNN
Health officials considered issuing a warning before deciding to recommend a pause of the Johnson &Johnson Covid-19 vaccine
CNN
U.S. pause on Johnson & Johnson vaccine could be felt the most in poor countries
LA Times
The dilemma in pausing J&J vaccine over risk of blood clots: Will it save lives or cost them?
LA Times
All Californians 16 and over can now get COVID-19 vaccine. Here’s how
LA TimesDiscover Related