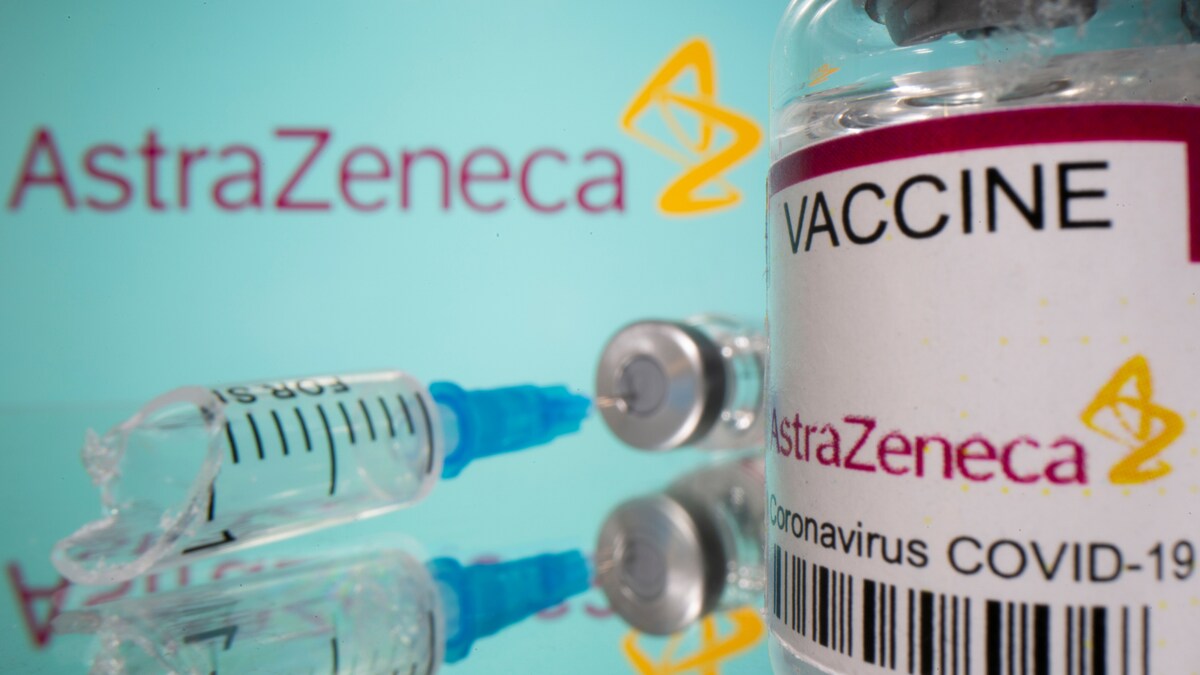
India to Participate in Global Trials to Assess Safety of Covishield among Cancer, HIV, Transplant Patients
News 18India will soon kick-start trials to assess the safety and immunity of AstraZeneca’s Covid-19 vaccine, dubbed Covishield in India, among unvaccinated immunocompromised adults, News18.com has learnt. According to the study planned on the global scale, previously unvaccinated immunocompromised adults will be enrolled in five disease cohorts — solid organ transplant, hematopoietic stem cell transplant, solid organ cancer patients receiving cytotoxic chemotherapy, HIV, auto-immune diseases and a group of healthy adults. The trial, which is The purpose of this Phase 4 study, according to the brief shared by the company with the country’s apex drug regulator, Central Drugs Standard Control Organisation, is to “demonstrate the safety and characterize the immunogenicity of AZD1222; AstraZeneca’s candidate ChAdOx1 vector vaccine against SARS-CoV-2 in previously unvaccinated immunocompromised adults”. Under the solid organ transplant category, the trial aims to enroll participants with heart, lung, kidney or liver transplants who are stable on immunosuppressants, whereas under cancer patients category, the participants with solid tumours who have a life expectancy of longer than three months will be recruited.
History of this topic

Health Talk | How eliminating polio has been one of the major successes of India's Universal Immunisation Programme
Hindustan Times
India to undertake mega study of covid-19 samples to help prepare for future pandemics
Live Mint
Global Pandemic Threat: 100 Days to Save Lives
Hindustan Times
With State prod, India can be world leader in vaccines
Hindustan Times
Accelerating Routine Immunization in India: Insights from Covid-19 Drive
The Quint
India urges G-20 countries to join vaccine research collaborative in wake of COVID vaccine inequity
The Hindu
India’s Winning Formula: Making Healthcare Affordable and Accessible with PPPs
News 18
India is 1 of 3 countries where confidence in children’s vaccines has increased: UNICEF report
The Hindu
India among countries with highest vaccine confidence. What about its neighbours
Hindustan Times
Explained | What are the problems with India’s clinical trials registry?
The Hindu
Serum Institute could resume Covishield production as Covid cases rise
Live MintIndia sees over 7,800 COVID cases in a day reported on April 12, 2023
The Hindu
The big tests of India’s healthcare ambitions, in 5 charts
Live MintActive COVID-19 cases in India rise to 21,179
The Hindu)
‘The Vial’ trailer out: Manoj Bajpayee narrates History TV18's documentary on India’s Covid-19 vaccine journey
Firstpost
New rules for flyers from 6 countries visiting India kicks in: 10 things to know
Live MintIndia to face ‘tsunami’ of chronic diseases like cancer: U.S.-based oncologist
The Hindu
\'India develops herd immunity; BF.7 variant may not be as serious as in China\'
Deccan Chronicle
Airports to randomly test 2%international flyers for Covid-19
Hindustan Times
Three reasons India will not see a Covid-wave like China’s
Hindustan Times
What Makes India's Covid Vaccination Programme A Global Best Practice In Public Health
News 18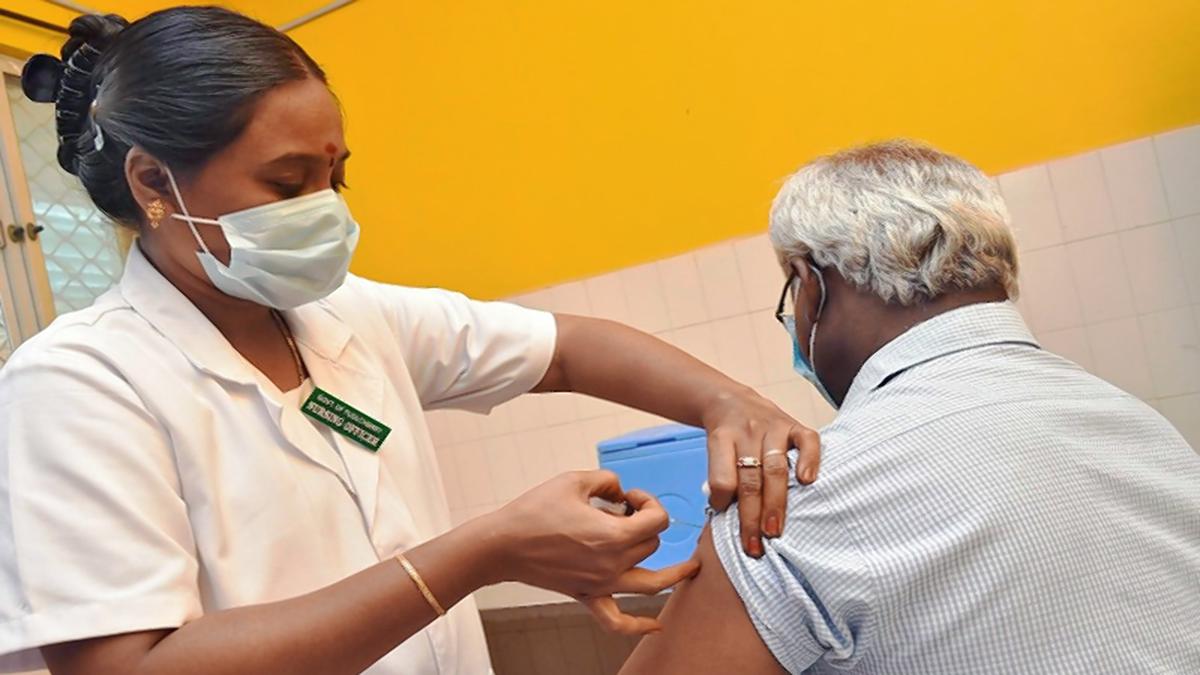
Health Ministry not to procure fresh COVID-19 vaccines; surrenders ₹4,237 crore from vaccination budget
The Hindu
Covid-19: India logs nearly 5,000 new infections, active cases drop below 45k
Live Mint
The outline of an essential global pandemic treaty
The Hindu
An End to Foreign-return Vaccine Woes: India to Offer Remaining Covid-19 Shots Domestically
News 18
How India Pursued Covid-19 Vaccine Diplomacy Amid Vaccine Nationalism By Rich Nations
ABP News
Why India Is Becoming One Of The Most Preferred Destination For Clinical Drug Trials
ABP News
Go For More Tests, Faster Vaccination: Worried by Uptick in Covid Graph, Centre's Advisory to 7 States
News 18)
India supplied 23.9 crore COVID-19 vaccine doses to 101 countries, UN entities: Centre
Firstpost
India reports 45 covid deaths, over 21,000 fresh cases in a day
Live Mint
Centre reviews Covid situation in states witnessing surge in cases, flags low level of testing
India TV News
Two billion Covid vaccine doses given in India, government says
The IndependentIndia crosses milestone of 200 crore vaccinations against COVID-19 in 18 months
The Hindu
DC Edit | A good move, as Covid cases up
Deccan Chronicle
Free Covid booster doses will create healthier country: PM Modi
India Today
India Reports 17,336 Fresh COVID-19 Cases in 24 Hours, 13 Fatalities
News 18
Over 42 lakh deaths in India prevented by COVID-19 vaccines in 2021: Lancet study
The Hindu
Jabs for Kids, Reducing Gap Between Doses for Those Travelling Abroad on Agenda as NTAGI Meets Today
News 18
As COVID-19 cases rise in north India, experts urge caution
The HinduBRICS grouping should work together to support vaccine endeavours: Health Minister
The Hindu
'Massive Participation': India's Covid Vaccine Drive Reaches Another Milestone as 75% Teenagers Get First Dose
News 18
Being ready: The Hindu Editorial on vaccination and India’s third COVID-19 wave
The Hindu
Coronavirus updates March 04, 2022
The Hindu92% of COVID-19 deaths in 2022 have been among unvaccinated: ICMR
The HinduCoronavirus live |
The Hindu
India Crosses 173.38 Crore Covid Vaccine Doses, Says Health Ministry, Mandaviya Congratulates 'Young India'
News 18)
World Cancer Day 2022: How COVID-19 impacted cancer care in India
Firstpost
India Seeing Fewer Covid Deaths; Centre Flags Maha, Delhi, UP Among States of Concern
News 18An epidemiologically sound testing strategy
The HinduDiscover Related





Government to launch 100-day nationwide TB campaign, denies shortage of anti-TB drugs
New Indian Express















)