Covid vaccine update: Lawsuits, probe shroud AstraZeneca vaccine trial in India over volunteer's illness
India TodayA 40-year-old volunteer, who was part of the third phase of clinical trial of the coronavirus vaccine being developed by AstraZeneca and Serum Institute of India and is undergoing trials in India, has claimed he suffered neurological symptoms following the dosage. While the volunteer has sent a legal notice to the vaccine makers and sought Rs 5 crore damages, SII has filed a defamation case and sought Rs 100 crore in damages. Indian volunteer seeks damages from AstraZeneca The AstraZeneca coronavirus vaccine is undergoing trials in India in its partnership with the Pune-based SII. A legal notice has been sent to Director General, ICMR, Drugs Controller General of India, Central Drugs Standard Control Organisation, CEO, AstraZeneca UK, Professor Andrew Pollard, Chief Investigator, Oxford Vaccine Trial and Vice-Chancellor of Sri Ramachandra Higher Education and Research. Speaking to India Today TV, a senior official at SII Pune hit back at the volunteer and said, "The drug controller office and health ministry office do not have any problem with the Serum vaccine as they know that the claims made by Chennai-based volunteer is not genuine.” Refuting the allegations as baseless, SII has sued the volunteer for Rs 100 crore in a defamation suit.
History of this topic

Covishield Side Effects: Doctors' Group Urges Govt to Review All Covid Vaccines
News 18
AstraZeneca withdraws Covishield pointing to low demand, but did rare side effects also play a role?
The Hindu
AstraZeneca's Covishield Showed Lowest Incidence of Side Effects in India Versus Globally, Says Top Govt Official
News 18
After AstraZeneca covid-19 vax recall worldwide, Serum says it stopped manufacturing jabs in Dec 2021
Live Mint
Stopped manufacturing Covishield since December 2021, says Serum Institute
Hindustan TimesAstraZeneca pulls its COVID-19 vaccine from the European market
Associated Press
AstraZeneca removes its Covid vaccine worldwide after rare and dangerous side effect linked to 80 deaths in Britain was admitted in court papers
Daily Mail
Covishield: Gujarat Cong Demands Compensation, BJP Claims Heart Attacks Not Linked to Vaccine
News 18
Only 7 Out 10 Lakh May Face Clotting Risk Due to Covishield: Former ICMR Scientist
News 18
AstraZeneca reaffirms Covid vaccine safety amid rare side effect concerns
India TV News
With State prod, India can be world leader in vaccines
Hindustan Times
Over 220 crore COVID vaccine doses administered across India, but less than 23 crore booster doses: Health Ministry data
The Hindu)
EU countries destroyed €4 billion worth of Covid-19 vaccines, reveals research
Firstpost
COVID-19 Update: India records 260 new cases, total active at 1,828
Live Mint
AstraZeneca strikes £880m deal to buy US vaccine maker amid questions over Covid jab
The Telegraph
The Vaccine War: A tale of Indian scientists' triumph over COVID-19 amid the Left's propaganda to undermine indigenous vaccine
Op India
Should you get the new COVID booster? The CDC is about to decide
LA Times
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
India-made mRNA vaccine priced at ₹2,292, will be available as a booster dose
The Hindu
India reports 95 new covid cases in a day
Live Mint
Axed Covid vaccine contract cost taxpayers £358m
The Independent
At 14,493, India records sharp dip in daily Covid tally, logs 801 new cases
Hindustan Times
Serum Institute of India to produce 5mn Covishield doses in two months
Hindustan Times
WATCH: SII chief Adar Poonawalla on vaccine availability, current Covid strain
Hindustan Times
India logs 9,111 new coronavirus infections, active cases climb to 60,313
Deccan Chronicle
Covid-19: India Reports Close to 11k Fresh Cases Again, Active Tally Exceeds 53,000
News 18
India restarts Covid vaccine production as new infections soar 30% in one day
The Independent
Serum Institute could resume Covishield production as Covid cases rise
Live MintActive COVID-19 cases in India rise to 21,179
The HinduIndia witnesses 3,016 fresh COVID-19 cases, highest in nearly six months
Deccan Chronicle
'Force to Reckon With in Near Future': Ex-ICMR DG Praises Govt’s 'Deliberate' Call to Give Free Covid Jabs
News 18
India records 1,300 new Covid cases, highest in 140 days
Hindustan Times
Active COVID-19 cases in country climb to 7,026
The Hindu
India records 1,134 new Covid cases, active tally crosses 7,000 mark
Hindustan Times)
‘The Vial’ trailer out: Manoj Bajpayee narrates History TV18's documentary on India’s Covid-19 vaccine journey
Firstpost
India records over 300 new Covid cases after 97 days
Hindustan Times
India saved 3.4mn people through Covid-19 vaccination drive: Report
Hindustan Times
Sliding vaccine sales, new costs, squeeze Moderna 4Q profit
Associated Press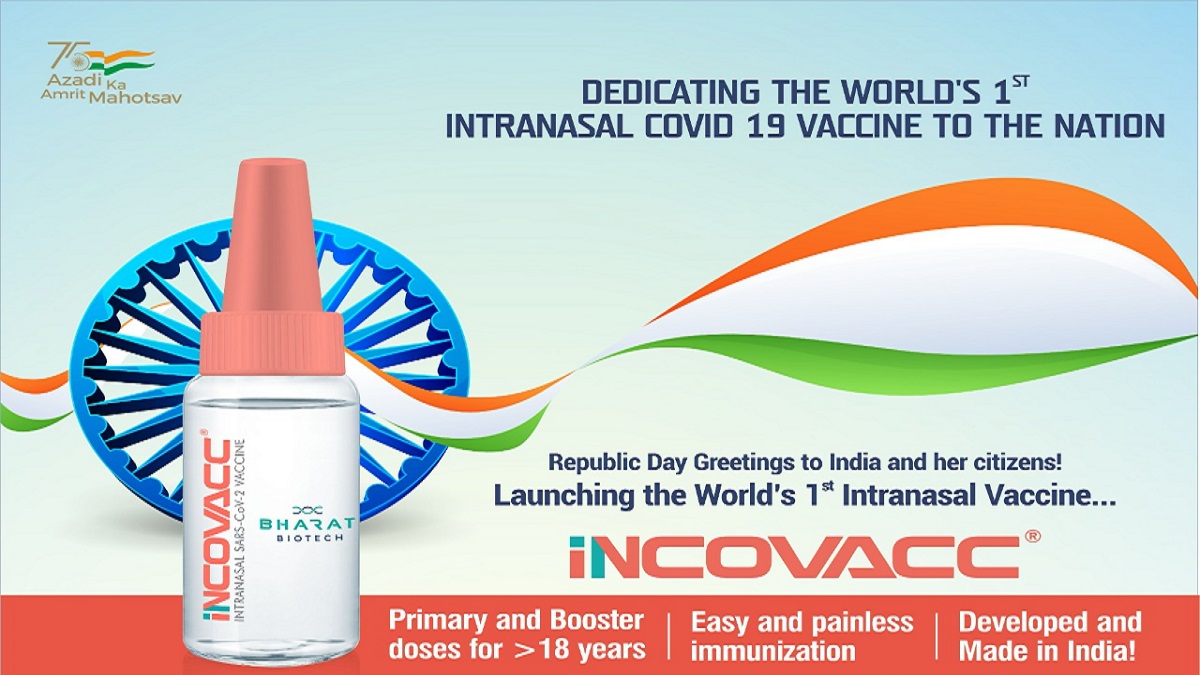
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News
COVID-19: Health Minister Mansukh Mandaviya to launch world's 1st 'Made-in-India' nasal vaccine today
India TV News
First Indian intranasal Covid vaccine by Bharat Biotech to be launched on Jan 26
India TV News
Pfizer CEO Cornered at Davos, India Saw Through Pharma Giant’s Promises Early to Not Bank on it
News 18
Covid-19: Active Cases in Country Dip to 2,149
News 18
100 Cr Defamation Suit | 'People Responsible For Saving Four Million Lives Being Branded As Mass Murderers': Serum Institute To Bombay High Court
Live Law)
COVID-19: Covovax vaccine to get approval as booster dose in 10-15 days, says Poonawalla
Firstpost
214 Fresh Covid-19 Cases, 4 Deaths in Last 24 Hours
News 18
No second Covid-19 booster dose required: Report
Live Mint
Biological E, Bharat Biotech Together Sitting on Stockpile of 250 Million Covid Vaccine Doses
News 18
Biological E, Bharat Biotech have stockpile of 250 million COVID vaccine doses
India TV News
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The HinduDiscover Related
















)








)