India Says WHO’s Info ‘Inadequate’ to Probe Cough Syrup-Linked Gambia Deaths, Seeks More Details
News 18India has sought more details from the World Health Organization to continue with its probe into the death of 66 children in The Gambia allegedly due to four made-in-India cough syrups. In an email response, Drugs Controller General of India V G Somani on Saturday said the Union Ministry of Health has constituted a committee of technical experts to examine and analyse the details of the adverse event reports and all related details shared by or to be shared by WHO and to recommend follow up action. Mentioning the observations, Dr Somani said, “The clinical features and the treatment received by the children as shared by WHO so far are inadequate to determine the aetiology.” He said that the details of initial illness, sign and symptoms, duration of anura in the cases, results of laboratory investigations conducted including various markers and parameters, specific investigations for diethylene glycol and ethylene glycol on critical samples of the patients, treatment received before and after hospitalization at the tertiary hospital in Gambia, treatment received before and after acute kidney injury was suspected and reasons thereof, names and brands of the drug formulations used in the treatment before and after hospitalization, their manufacturers, their expiry other relevant information in each of the cases, are necessary, news agency PTI reported. The Union Health Ministry on Wednesday formed the four-member panel of experts to examine the details and adverse event reports received from the WHO on the deaths of 66 children in Gambia being linked potentially to the four cough syrups made by the Sonipat unit of Maiden Pharmaceuticals.
History of this topic

6% of cough syrup samples fail export quality test
The Hindu)
Vantage | Why poor quality cough syrups may poison India's reputation too
Firstpost
Gambia parents ‘fight for children’ in landmark trial on India syrup deaths
Al Jazeera
Two labs in India take on the bulk of testing for cough syrup samples for export
The Hindu)
India finds 2 more toxic cough syrups: What we know about them
Firstpost
The lowdown on ‘substandard’ cough syrups
The Hindu
'India will never bargain on quality of medicines': Health minister Mandaviya on cough syrup row
Hindustan Times
No tolerance on spurious meds, 71 firms issued notices: Mandaviya on cough syrup row
Deccan Chronicle
Safety first: on Indian pharma products and drug safety
The Hindu
India's Claim to ‘Pharmacy of the World’ in Limbo, Fixing Quality of Drugs an Emergency - News18
News 18
Test cough syrups meant for exports on priority: DCGI
Hindustan Times
India considers policy change after cough syrup deaths: PM Modi's office
The Hindu
Gambia-Uzbekistan Fallout: India to Put a System of Checks at Govt Labs Before Exporting Cough Syrups
News 18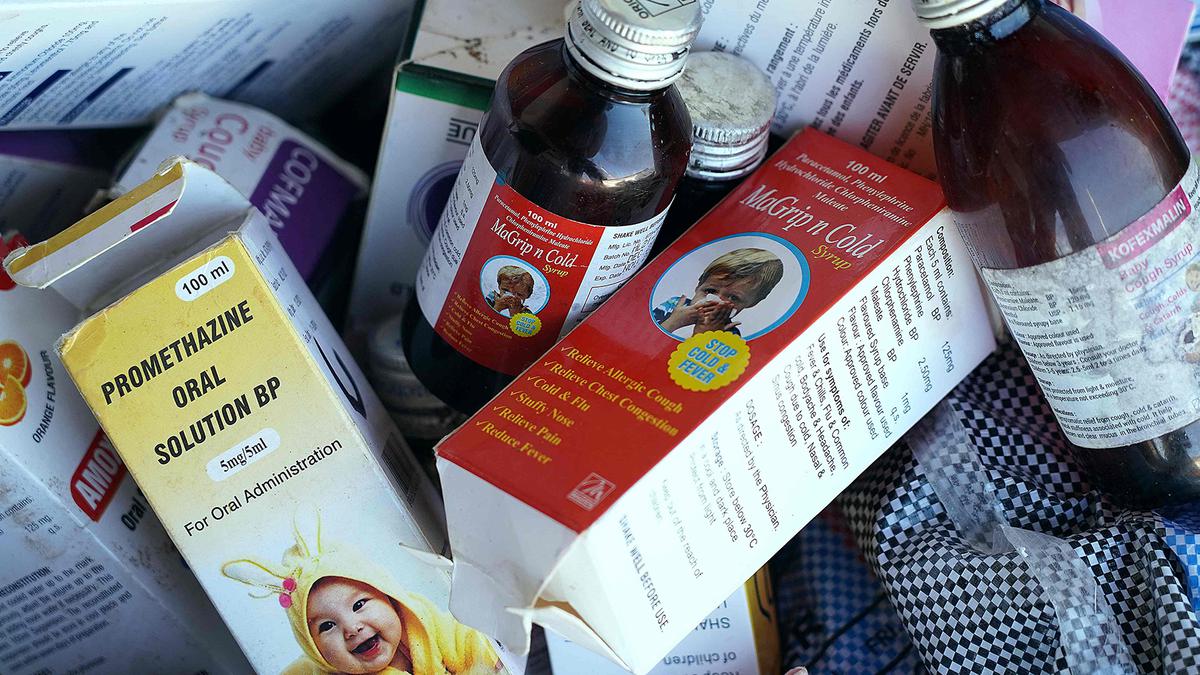
Indian cough syrup: mystery middleman may be new clue
The Hindu
Indian cough syrup: Mystery middleman may be new clue
Live Mint)
WHO issues alert for another India-made cough syrup: Why are these drugs so deadly?
Firstpost
Guaifenesin: WHO flags India-made ‘contaminated’ cough syrup with ‘unacceptable amounts’ of toxic chemicals
The IndependentContaminated syrups manufactured in India reported in WHO’s Western Pacific region
The Hindu
Cough Syrup Deaths in Gambia, Uzbekistan: 18 Indian Drug Makers Lose Licence in Big Clean-Up
News 18
Licences of six cough syrup makers in Maharashtra suspended for violation of rules
The Hindu
Cough Syrup Death Cases: US CDC Blames Indian Pharma Firm for Demise of Gambia Kids; 3 Held in Noida Over Uzbekistan Incident
News 18
U.S.-CDC probe into cough syrup deaths in The Gambia pins blame on Indian manufacturer
The Hindu
Cough Syrup Row: Maharashtra Govt Acted against 27 Pharma Firms, Says Minister
News 18
Gambia, Uzbekistan Effect? Modi Govt Plans Two-day 'Chintan Shivir' of Drug Regulators, Policymakers
News 18WHO calls for action to protect children from contaminated medicines after cough syrups deaths
The Hindu)
WHO urges 'immediate action' after 300 children die due to cough syrup
Firstpost
Cough Syrup deaths: WHO investigating links between manufacturers in India
Live Mint
WHO calls for action to protect children from contaminated medicines after cough syrups deaths
India TV News
WHO Urges 'Immediate and Coordinated Action' on Contaminated Medicines After Child Deaths
News 18
Indonesia court hearing into children killed by toxic cough syrup
Al Jazeera
Uzbekistan, Gambia Cough Syrup Cases: 'Concerned' Commerce Ministry Advises Thorough Probe - News18
News 18
Behind the Indian cough syrup that killed many in Gambia and Uzbekistan
The Hindu
'Substandard...': WHO warns against use of cough syrups linked to Uzbek deaths
Hindustan Times
India cough syrup linked to Uzbekistan deaths ‘substandard’: WHO
Al Jazeera
At the precipice of shame: The Hindu Editorial on The Gambia and Uzbekistan cough syrup deaths
The Hindu
Marion Biotech, cough syrup makers linked to Uzbek deaths, halts all production
Hindustan Times
Noida firm stops syrup blamed for 11 kid deaths
Deccan Chronicle)
‘Closely monitoring probe’: Indian embassy on death of 18 kids after consuming cough syrup in Uzbekistan
Firstpost
BJP, Cong spar over cough syrup related deaths in Uzbekistan, Gambia
Deccan Chronicle
India seeks details of Uzbekistan’s investigation into cough syrup
Hindustan Times
Congress, BJP trade barbs over cough syrup-related deaths of children in Uzbekistan and Gambia
The Hindu
Uzbek cough syrup deaths: Mandaviya asks CDSCO to probe matter, samples sent for testing
Live Mint
Cough syrup deaths | India seeks details from Uzbekistan on investigations
The Hindu
Uzbekistan Syrup Deaths: BJP, Congress Lock Horns Over India's 'Pharmacy to the World' Title
News 18
Indian pharma under scrutiny again after 18 Uzbek child deaths
Al Jazeera
18 children dead due to cough syrup made by Indian firm, says Uzbekistan
The Hindu
Uzbekistan says 18 children die after consuming India-made syrup
Al Jazeera)
WHO drew premature link between children deaths in Gambia, India-made cough syrups: DCGI
Firstpost
WHO drew premature link between Gambia children deaths, India-made cough syrups: DCGI
Deccan Chronicle
Samples Not Contaminated, Meets Standards: Centre Slams WHO Over Premature Links Between Children Deaths in Gambia and India-made Syrups
News 18Discover Related





)