Explained | What are the problems with India’s clinical trials registry?
The HinduThe speedy approval of Covid-19 vaccines during the SARS-CoV-2 pandemic spotlighted the importance of clinical trials. Hosted with the Indian Council of Medical Research’s National Institute of Medical Statistics, the CTRI is a free, online public-record system to register clinical trials being conducted in India. The CTRI is one of 17 public trial registries under the International Clinical Trials Registry Portal, along with being recognised as a primary registry by the World Health Organisation. Variations in the classification of organisations – CTRI provides the option to classify ‘primary sponsors’ under several categories, like contract research organisations, the pharmaceutical industry, and research institutions. Some trials do not follow the rules or may break the law – All clinical trials in India must be registered on CTRI in India, even if the trial is also registered elsewhere.
History of this topic

Health Talk | How eliminating polio has been one of the major successes of India's Universal Immunisation Programme
Hindustan Times
India to undertake mega study of covid-19 samples to help prepare for future pandemics
Live Mint
Global Pandemic Threat: 100 Days to Save Lives
Hindustan Times
Health Talk | Why India waiving off local clinical trials for globally approved new drugs is good news
Hindustan Times
Clinical trial: Government announces waiver for several drugs approved from select countries
The HinduIn India, all vaccine-related AEFI are routinely monitored and COVID is no exception, says health expert
The Hindu
COVID-19 Update: India records 260 new cases, total active at 1,828
Live Mint
Burying the fallacy of vaccine sceptics
Hindustan Times
Accelerating Routine Immunization in India: Insights from Covid-19 Drive
The QuintIs India ready for Controlled Human Infection Studies? | In Focus podcast
The Hindu
Welcome development: The Hindu Editorial on the ICMR and Controlled Human Infection Studies
The Hindu
Govt Eases Covid Guidelines for International Travellers
Deccan Chronicle
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The HinduClinical Trials: What is it?
The Hindu
India urges G-20 countries to join vaccine research collaborative in wake of COVID vaccine inequity
The Hindu
Debunking myths and misconceptions around immunisation or vaccination in India
Hindustan Times
India is 1 of 3 countries where confidence in children’s vaccines has increased: UNICEF report
The Hindu
'Force to Reckon With in Near Future': Ex-ICMR DG Praises Govt’s 'Deliberate' Call to Give Free Covid Jabs
News 18
India records 1,134 new Covid cases, active tally crosses 7,000 mark
Hindustan TimesIndia to face ‘tsunami’ of chronic diseases like cancer: U.S.-based oncologist
The Hindu
‘Erroneous’: Centre dismisses Covid vaccine's ‘multiple side effects’ report
Hindustan Times
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The Hindu
Holistic data analysis, upgraded vaccines to determine India's future battle with Covid: Epidemiologist Dileep Mavlankar
India Today
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News
Covid not over yet; need to be alert, increase booster doses uptake: Mandaviya tells Parliament
Live Mint
Health Ministry asks States to hike genome sequencing for the SARS-CoV-2 coronavirus
The Hindu
COVID-19 vaccine: How well does India’s adverse reaction tracking system work? | In Focus podcast
The Hindu
What Makes India's Covid Vaccination Programme A Global Best Practice In Public Health
News 18
Covid-19: India logs nearly 5,000 new infections, active cases drop below 45k
Live Mint)
Coronavirus Update: India registers decrease in new Covid-19 cases
Firstpost
An End to Foreign-return Vaccine Woes: India to Offer Remaining Covid-19 Shots Domestically
News 18
Why India Is Becoming One Of The Most Preferred Destination For Clinical Drug Trials
ABP News
Travelling to India from abroad? Govt mulls doing away with this rule
Live Mint
Covid-19 update: India sees another rise with 19,893 cases in 24 hrs
Live Mint
India reports nearly 22,000 fresh covid cases, 60 deaths in last 24 hours
Live Mint
India reports 45 covid deaths, over 21,000 fresh cases in a day
Live Mint
India crosses 200-cr vaccine doses landmark, PM writes to vaccinators lauding their efforts
India TV News
Centre reviews Covid situation in states witnessing surge in cases, flags low level of testing
India TV News
Two billion Covid vaccine doses given in India, government says
The IndependentIndia crosses milestone of 200 crore vaccinations against COVID-19 in 18 months
The Hindu
DC Edit | A good move, as Covid cases up
Deccan Chronicle
India Reports 17,336 Fresh COVID-19 Cases in 24 Hours, 13 Fatalities
News 18
Over 42 lakh deaths in India prevented by COVID-19 vaccines in 2021: Lancet study
The Hindu
Focus on adequate testing, surveillance: Mandaviya at Covid review meeting
India TV News
Bharat Biotech Nasal Covid Vaccine's Phase 3 Trials Completed
News 18
No Haste In Granting Emergency Use Authorization To Covaxin & Covishield: Supreme Court
Live Law
In haste: The Hindu Editorial on vaccinating children against COVID-19
The Hindu
India to Participate in Global Trials to Assess Safety of Covishield among Cancer, HIV, Transplant Patients
News 18
55% citizens in favour of govt expanding Covid booster dose programme: Survey
India TodayDiscover Related



)




Government to launch 100-day nationwide TB campaign, denies shortage of anti-TB drugs
New Indian Express





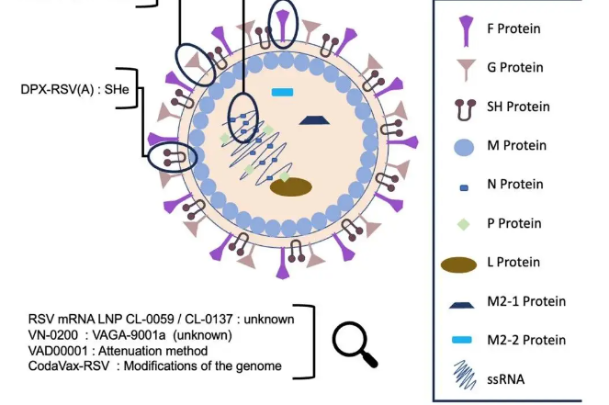





)
















)