Coronavirus | U.S. gives full approval to antiviral remdesivir to treat COVID-19
The HinduThe U.S. Food and Drug Administration on Thursday granted full approval to the antiviral drug remdesivir as a treatment for patients hospitalized with COVID-19, after conditional authorization was given in May. However, other treatments have received authorization for emergency use, though that approval can be revoked once the public health emergency sparked by the coronavirus pandemic is over. “The FDA is committed to expediting the development and availability of COVID-19 treatments during this unprecedented public health emergency,” said FDA Commissioner Stephen Hahn. “Today's approval is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the Covid-19 pandemic.” Europe and other countries such as Canada also have granted temporary approval for the use of remdesivir.
History of this topic
FDA revokes authorization for key anti-COVID drug, a blow for vulnerable Americans
LA Times
Remdesivir Could Reduce Covid Mortality if Given Early : Study
News 18Oral pill for COVID-19 approved by regulator
China Daily
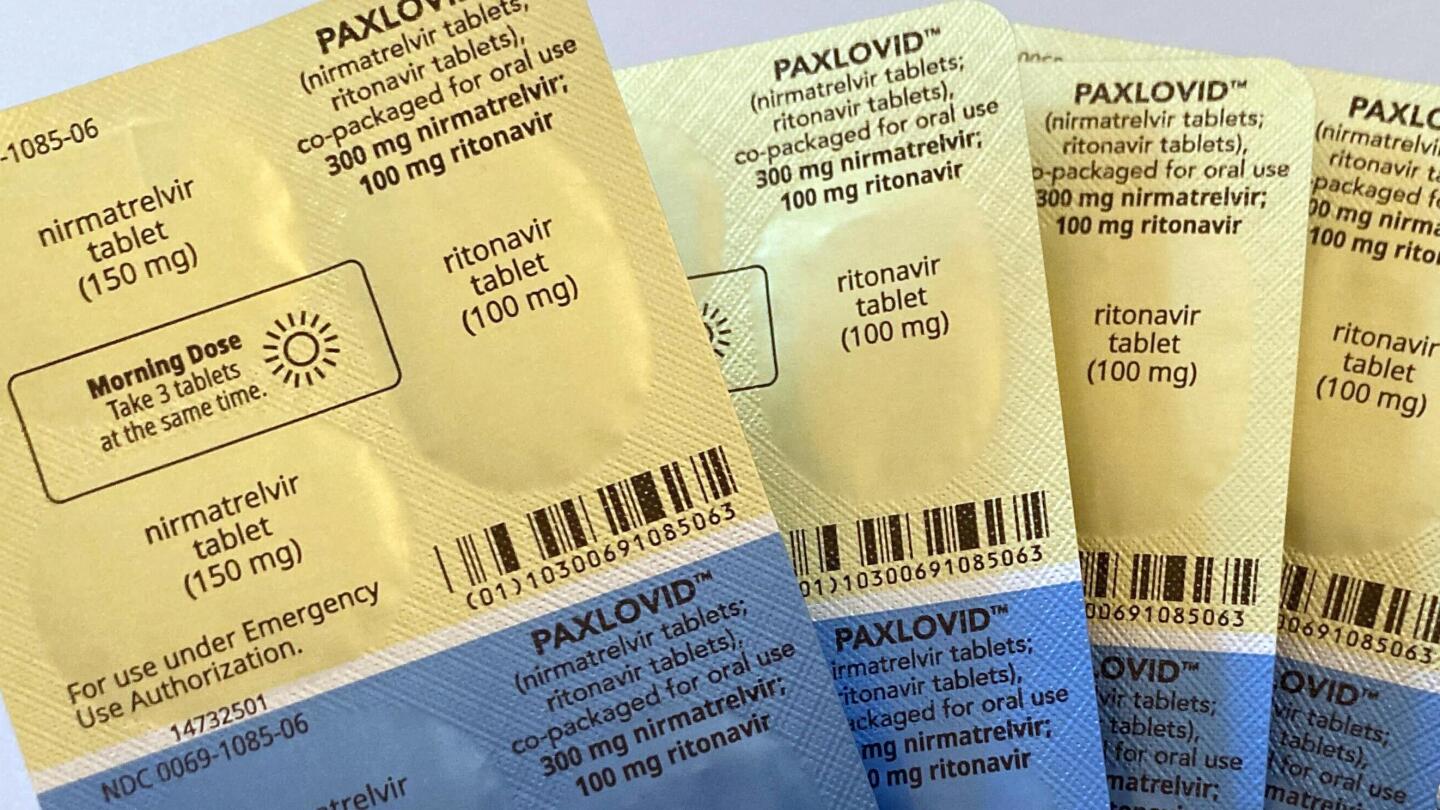
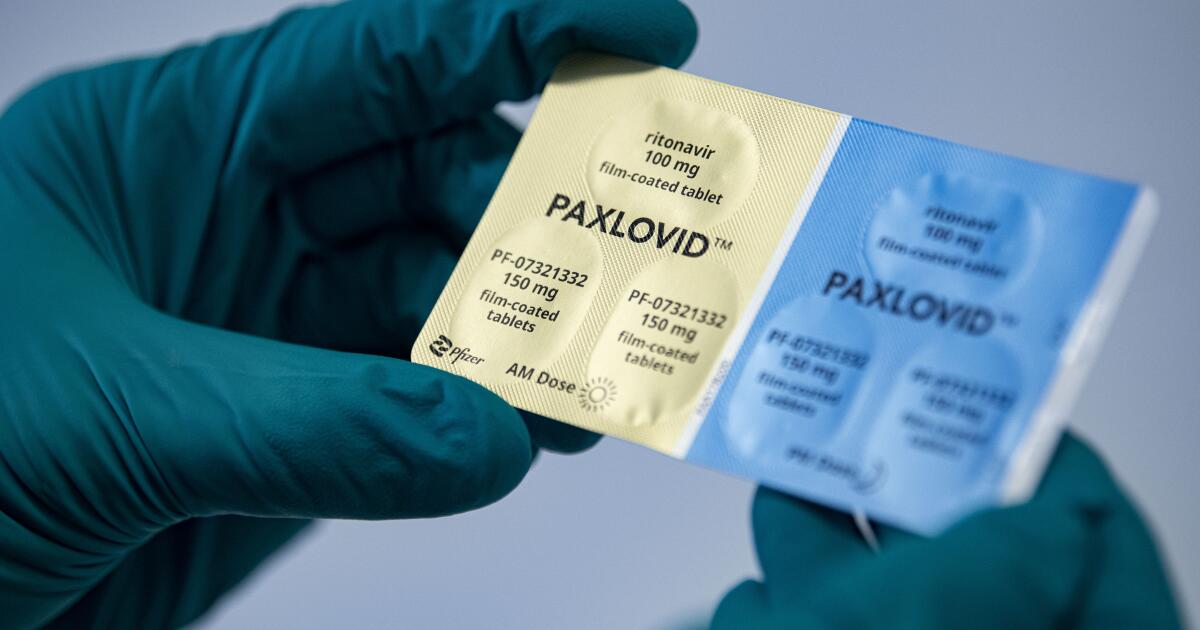
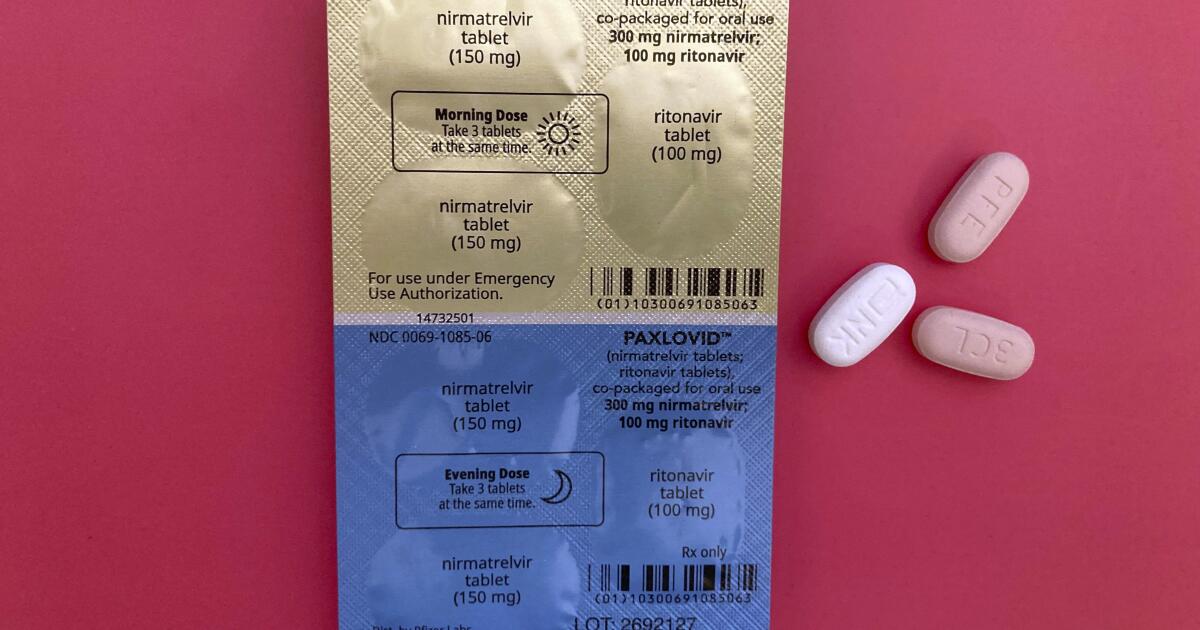
WHO recommends Pfizer's pill Paxlovid for mild Covid cases
India Today
Health Matters: Dear Drug Regulator, Silence is Not Always Golden, Esp on WHO Pausing Covaxin Supply
News 18
Why Remdesivir’s Manufacturing Licence was Not Given to Pharma PSUs When People Were Battling Second Wave: Parl Panel
News 18
Health Matters: Don't Feel Guilty About Not Finding Remdesivir for Your Loved One. It Hardly Works
News 18)
New COVID-19 treatment rules: India cuts down use of Remdesivir, Tocilizumab, multivitamins, steroids
Firstpost
India Cuts Use of Remdesivir, Tocilizumab, Multivitamins, Steroids in Covid-19 Treatment
News 18
A shot in the arm: The Hindu Editorial on new modes of COVID-19 treatment
The Hindu
Pfizer Covid-19 antiviral pill: Biden says he’s ‘encouraged by the promising data’
CNN
Merck asks EU drug regulator to authorise its Covid-19 pill
Hindustan Times
EU regulator recommends Merck’s COVID-19 pill for emergency use
Al Jazeera
Pfizer says COVID-19 pill cut hospital, death risk by 90%
Associated Press
Molnupiravir: UK authorizes Merck/Ridgeback Biotherapeutics’ antiviral to treat mild-to-moderate Covid-19
CNN
UK approves Merck’s antiviral COVID-19 pill in world first move
Al Jazeera
Merck seeks first US authorization for Covid-19 tablet
Live Mint
Merck seeks FDA emergency use authorization for experimental antiviral Covid-19 treatment molnupiravir
CNN
Merck seeks US authorisation for pill to treat COVID
Al Jazeera
New coronavirus anti-viral med is “welcome, but not a miracle cure”: Virologist
NL Times
Merck Covid pill: What would an antiviral pill mean for the fight against Covid-19?
CNN
WHO Tests 3 Drugs for Treating Coronavirus Infection
News 18
Remdesivir, HCQ have no antiviral effects: WHO study
India TV News
US to spend $3.2B on treatments for COVID-19, other viruses
Associated Press
Biden administration will spend $3.2 billion on development of antiviral pills for treating COVID-19
Daily MailDoctors welcome India’s COVID treatment protocol for children
The Hindu)
Don't Use Remdesivir, Limit CT Scan, Steroid: Centre's Covid Treatment Guidelines for Kids
News 18)
DGHS issues COVID-19 treatment guidelines: Here is what document recommends on Remdesivir, Tocilizumab
Firstpost)
Govt Issues Advisory for Rational Use of Remdesivir in Covid-19 Treatment
News 18Exercise extreme caution in Remdesivir use: Centre
The Hindu
DGHS drops Ivermectin, Doxycycline from Covid-19 treatment; ICMR rules unchanged
India Today)
No evidence of Remdesivir's effectiveness, might be dropped as COVID-19 treatment: Dr DS Rana
Firstpost
Remdesivir Sale To Be Shifted To Chennai Nehru Indoor Stadium
The Quint
Coronavirus Fact Check: Is Remdesivir life saving drug in COVID19, can it be given to patients at home?
India TV News
When to use Remdesivir, Ivermectin? India's Covid treatment protocol and what experts say
India Today)
Zydus Cadila Prices Covid-19 Medicine 'Virafin' at Rs 11,995 Per Dose
News 18
Families of Covid patients struggle to get Remdesivir in Tamil Nadu amid shortage
India Today)
What Widespread Recommendation of Remdesivir for COVID-19 and Its Shortage Teach Us
News 18)
COVID-19 Treatment: Cipla to manufacture and sell Eli Lilly's baricitinib in India
News 18)
Covid-19: Remdesivir Allocation Made Up to May 16 to Ensure Availability, Says Union Minister
News 18
Health Ministry's advisory for Covid patients in home isolation, their caregivers | All you need to know
India Today
Pfizer may have an anti-viral COVID-19 pill available by the end of the year
SalonDoctors divided over use but scramble for Remdesivir continues
The Hindu
American biopharmaceutical company Gilead announces steps to expand Remdesivir availability in India
India TV News
Russian firm awaits government approval to ship remdesivir to India
India TodayCoronavirus | Counter set up at KMC to sell Remdesivir
The Hindu)
COVID is a 'mild disease', less than 5% of patients need ventilator, says AIIMS chief Randeep Guleria
Firstpost)
Centre to Ramp UP Remdesivir Production From 38.80 Lakh Units Per Month to 74 Lakh Units Per Month From May: MHA
News 18Centre issues updated clinical guidance for management of COVID-19 patients
The Hindu)
'Not a Life-Saving Drug': ICMR Suggests Safe Use of Remdesivir in Covid-19
News 18Discover Related
























)