Explained: How the Moderna vaccine stacks up against Pfizer-BioNTech's
India TodayUS regulators authorized Moderna Inc's Covid-19 vaccine for emergency use on Friday, a week after granting the first US authorization to Pfizer Inc and BioNTech SE's coronavirus shot. Very few participants who received the vaccine in either trial got sick with COVID-19 and almost none got seriously ill. Data the companies have submitted to the Food and Drug Administration suggest that they start offering partial protection against Covid-19 around two weeks after recipients receive their first dose. While the vaccines have not been compared head-to-head, Moderna's vaccine appears to be associated with slightly more severe cases of fatigue, headache and fever in the day or two following the second shot, especially in people younger than 65. Britain's medical regulator has said anyone with a history of anaphylaxis, or severe allergic reactions to a medicine or food should not get the Pfizer/BioNTech Covid-19 vaccine.
History of this topic

Results are looking promising for a combined COVID and flu vaccine. Here’s how it could benefit public health
New Indian Express
Updated COVID vaccines will be available later this week, recommended for everyone six months and up
Salon
US FDA authorizes Pfizer-BioNTech, Moderna's updated Covid shots
India Today
Should you get the new COVID booster? The CDC is about to decide
LA Times
Moderna says updated COVID vaccine is effective against ‘highly-mutated’ BA.2.86 variant
Hindustan Times
US renews push for COVID boosters as data show they protect
Associated Press
Pfizer, Moderna launch trials to track whether issues arise YEARS after getting their vaccines
Daily Mail
Covid-19: Pfizer faces backlash for creating pro-vaccine Marvel comic
Daily Mail
UK Approves Pfizer Bivalent Covid Vaccine Ahead of Booster Drive
News 18
US CDC endorses updated Covid boosters, shots to begin soon
India Today
Moderna sues Pfizer/BioNTech for patent infringement over COVID-19 vaccine
The Hindu
Moderna Sues Pfizer, BioNTech For Patent Infringement Over Its mRNA Covid Vaccine
ABP News
Moderna sues Pfizer, BioNTech for patent infringement over Covid-19 vaccine
Live Mint
Moderna sues rival COVID-19 vaccine makers Pfizer and BioNTech
Al Jazeera
Tweaked COVID boosters close but how much will they help?
Associated Press
British regulator 1st to OK Moderna’s updated COVID booster
Associated Press
US to roll out COVID vaccines for youngest children next week
Al Jazeera
US Approves Pfizer, Moderna Covid-19 Vaccines for Youngest Children
News 18
Covid Vaccines For Children Under-Five Received US FDA Nod
News 18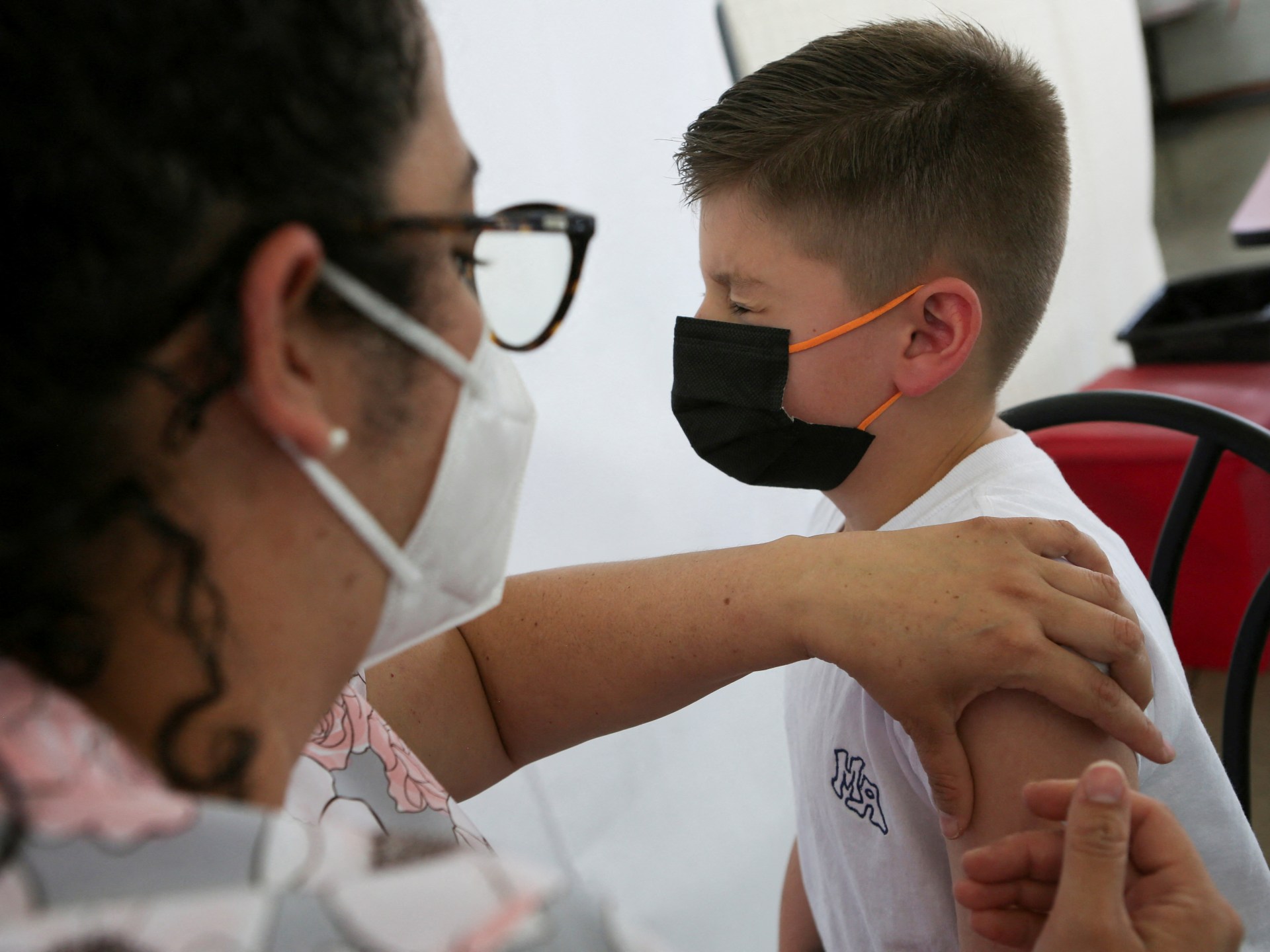
US: FDA authorises COVID-19 vaccines for children under 5
Al Jazeera
US approves first COVID-19 shots for infants and preschoolers
India TV NewsAmerica's FDA panel backs Moderna and Pfizer COVID-19 vaccines for children under five. Here's what that means
ABC
US Panel Recommends Moderna Covid Vaccine for Children Six and Up
News 18
FDA advisers back Moderna’s COVID-19 vaccine for older kids
Associated Press
FDA advisers vote in favor of authorizing Moderna Covid-19 vaccine for ages 6-17
CNN
Pfizer’s COVID-19 shot appears effective in kids under 5, FDA says
LA Times
FDA advisers to weigh expanding Covid-19 vaccines to younger kids
CNN
US FDA Says Moderna Covid Vaccine Effective For Children Under Five Years
News 18
FDA finds Moderna’s Covid-19 vaccine is safe and effective in younger kids
CNN
Marathon US hearings to decide fate of COVID shots for tots
Associated Press
Moderna says updated COVID shot boosts omicron protection
Associated Press
Moderna says its updated COVID vaccine boosts protection against Omicron
LA Times
Moderna Delays Covid Vaccine Deliveries to EU by Several Months
News 18
Pfizer completes EUA submission for Covid-19 vaccine for youngest kids
CNN
Fourth Covid-19 vaccine dose provides strong immunity boost: UK study
India TV News
Netherlands rejects Moderna Covid vaccine for children under 12
NL Times
Covid-19: US FDA approves another Pfizer, Moderna booster dose for age 50 and up
India Today
What you need to know about COVID vaccines for little kids
LA Times
Moderna says its low-dose COVID shots work for kids under 6
Associated Press)
Moderna says its low-dose COVID shots work for kids under six
Firstpost
Side Effects From mRNA Covid Vaccines Usually Mild And Temporary, Large US Study Finds
ABP News
Moderna asks FDA to authorize 4th COVID-19 dose for all adults
LA Times
EU clears Moderna shot for young kids and Pfizer for boosters
LA Times
Pfizer vaccine less effective against Covid19 in kids aged 5-11: Study
India TV News
CDC backs Moderna COVID-19 shots after full US approval
Associated Press
A different COVID-19 vaccine debate: Do we need new shots?
LA Times
US gives full approval to Moderna’s COVID-19 vaccine
Associated Press
Moderna's COVID-19 vaccine receives full FDA approval for Americans aged 18 and older
Daily Mail
U.S. gives full approval to Moderna’s COVID-19 vaccine
LA Times
Moderna starts trial of Omicron-specific COVID vaccine booster
Al JazeeraDiscover Related







)