Entod eye drops’ licence junked for misleading claims, overselling
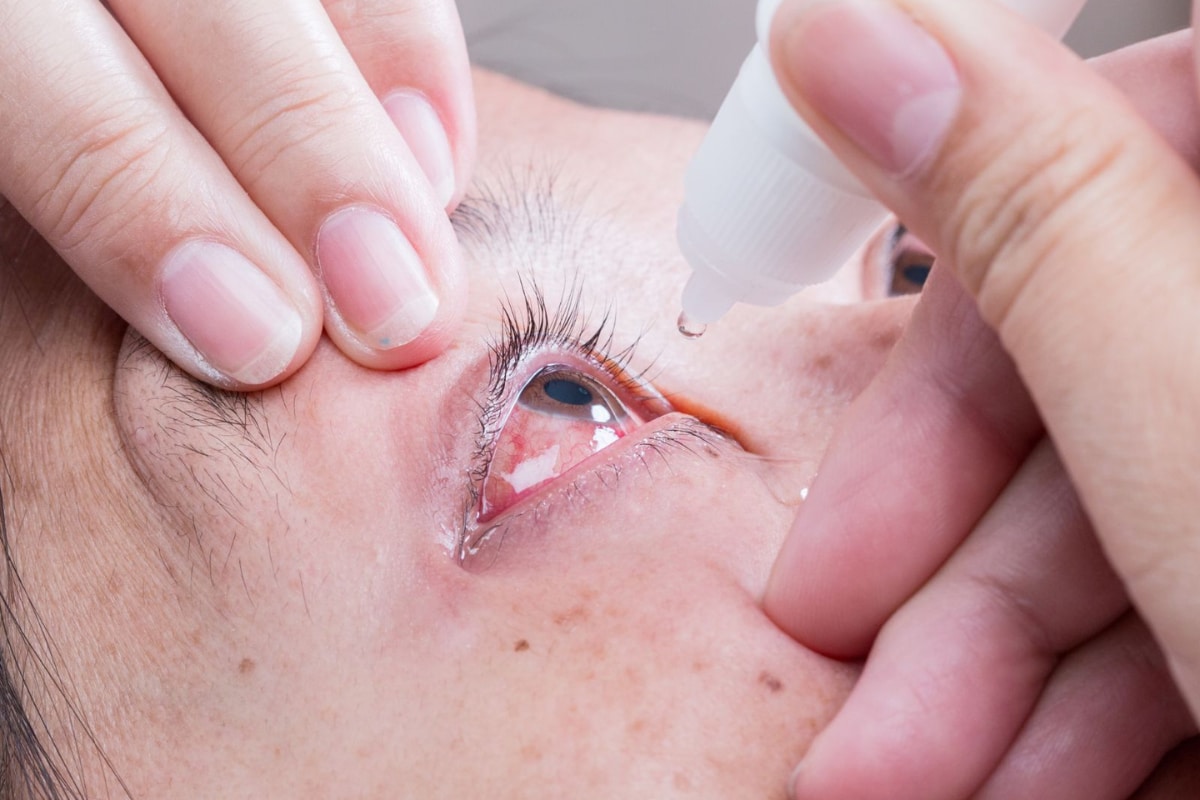
Hindustan TimesThe national drugs regulator has suspended its approval for Mumbai-based Entod Pharmaceuticals to make and sell its “PresVu” eye drops for overselling the product’s claimed benefits, which included reducing a presbyopia patient’s dependency on reading glasses. In an order issued on Tuesday, the Drugs Controller General of India said it issued permission on August 20 to manufacture and market Pilocarpine Hydrochloride Ophthalmic Solution, the chemical name of PresVu, to treat presbyopia in adults, permission which it was suspending till further orders. “… considering the public interest, the permission dated 20.08.2024 issued to manufacture and market of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% is hereby suspended till further order under the provisions of rule 84 of the New Drugs and Clinical Trials Rules, 2019, of the Drugs and Cosmetics Act, 1940,” read Drugs Controller General, Rajeev Singh Raghuvanshi’s letter. Among concerns the regulator raised include the company promoting it as the “first eye drop in India designed to reduce the need for reading glasses.” “In this regard you are informed that the pilocarpine hydrocholoride ophthalmic solution has not been approved for any such claim that it is designed to reduce the need for reading glasses.” In response to the claim “this eye drop offers a non-invasive option that can enhance near vision without the need for reading glasses”, the company submitted that in the clinical trial conducted, subjects did not wear glasses to participate.
History of this topic

Eye drop not intended to replace reading glasses, ENTOD pharma clarifies after DCGI suspension
New Indian Express
Drug regulator suspends product PresVu over unauthorised promotion
Hindustan Times
Drug regulator suspends manufacturing and selling of Entod pharma's eye drops over unapproved claims
India TV News
Drug regulator suspends pharma firm from manufacturing eye drops claiming to replace reading glasses
New Indian Express
DCGI suspends Entod’s marketing approval for PresVu eye drops amid misuse concerns
Hindustan Times
Pres Vu eye drops: Centre suspends permission for manufacture and marketing of eye drops claiming to do away with reading glasses
The Hindu
Say goodbye to reading glasses, read to know more
Deccan Chronicle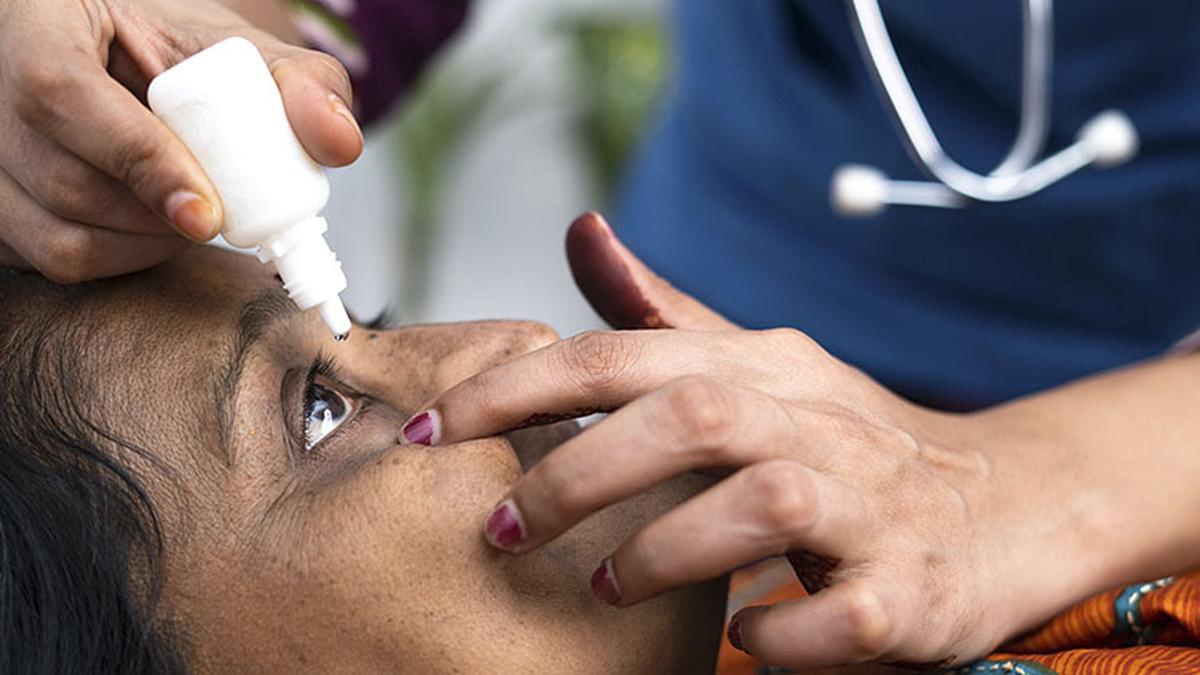
Unethical and false presentation of facts’ by Entod Pharmaceuticals on PresVu eye drops, say official sources
The Hindu
Expert opinion: Can this new made-in-India eye drop actually help you get rid of your glasses?
Hindustan Times
Eye Drops To Eliminate Need For Reading Glasses to Hit The Indian Market by October
News 18Discover Related











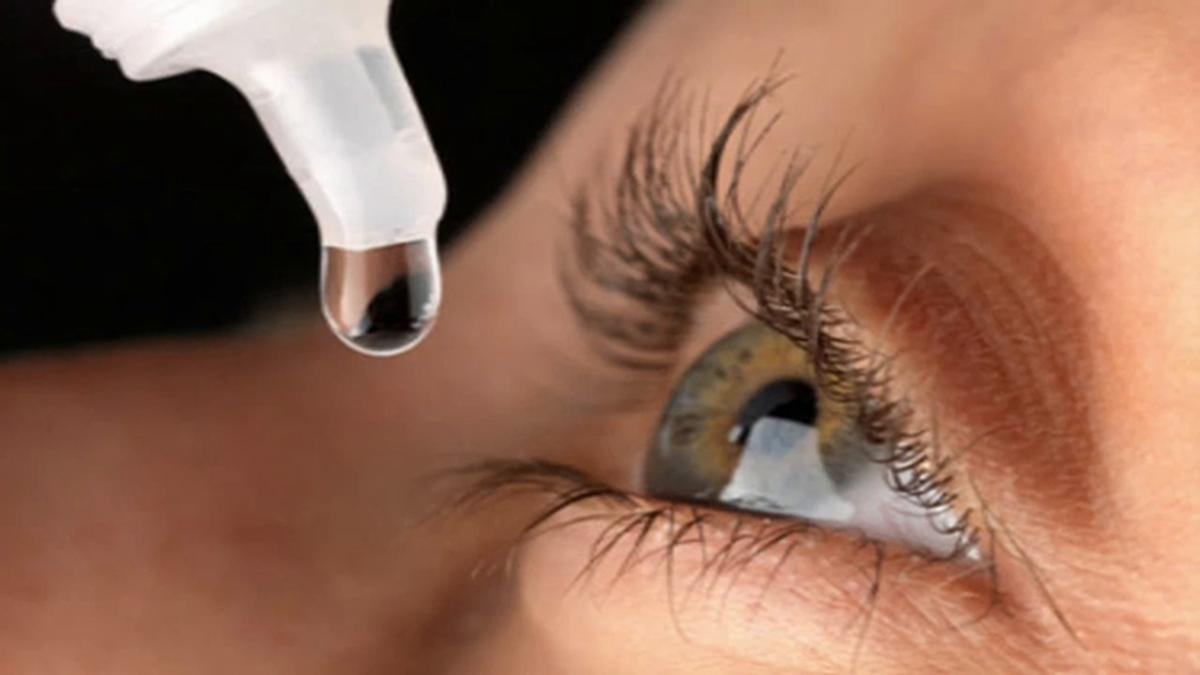
Guj HC notice to state govt on 17 alleged cases of vision loss post cataract surgery
Hindustan Times




)