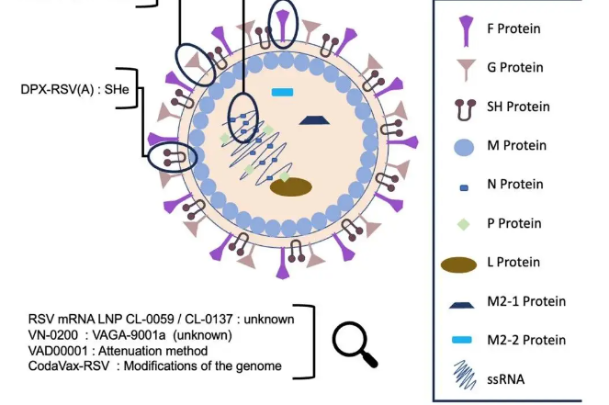
Zydus Cadila to commence phase II clinical trials of COVID-19 vaccine from Aug 6
The HinduDrug firm Zydus Cadila on Wednesday said the phase I clinical trial of its COVID-19 vaccine candidate, ZyCoV-D, has been completed and it will commence phase II clinical trials from August 6. “The company reports that the doses of the vaccine administered to healthy volunteers in the phase I clinical trial, which began on July 15, 2020, has been well tolerated,” Cadila Healthcare, the listed entity of the group, said in a regulatory filing. We now begin the phase II clinical trials and look forward to evaluating the safety and immunogenicity of the vaccine in a larger population, he added. It got approval a few days after India’s first indigenous COVID-19 vaccine candidate COVAXIN, developed by city-based Bharat Biotech in collaboration with Indian Council of Medical Research and National Institute of Virology, got the nod for human clinical trials from the Drug Controller General of India Shares of Cadila Healthcare were trading at ₹407.20 apiece on BSE, up 1.08% from its previous close.
History of this topic

As Indians Choose to Ignore Booster Shots, Pace of Vaccine Testing Slows Down at Himachal Lab
News 18
India clears 2 vaccines for kids under 12 years
Hindustan Times
Covovax, Corbevax, ZyCov-D Not Rolled Out Months After DCGI Nod; Now is Right Time, Say Experts
News 18
Only Covaxin for 15-18 year-olds, booster shots 9 months after second dose | Govt guidelines
India Today
India's First Needle-free Covid Vaccine to Hit Market: Here's All About the 3-dose ZyCov-D Jab
News 18
ZyCoV-D, India’s First Covid Vaccine for Children Aged 12+, to be Administered Among Adults in 7 States First
News 18
2,37,530 Doses of Needle-free Covid Vaccine ZyCoV-D Released for Use from CDL after Testing
News 18
Centre Orders 1 Crore Shots of Zydus Cadila's Covid-19 Vaccine at Rs 265 Per Dose
News 18
Centre places purchase order for one crore doses of Zydus Cadila’s Covid vaccine
Live Mint
Zydus Cadila Agrees to Reduce Covid Vaccine Price to Rs 265 a Dose, Final Decision Soon: Sources
News 18
Zydus Cadila agrees to reduce its Covid vaccine price to ₹265 a dose, final decision soon: Report
Live Mint
60 lakh ZyCoV-D's Needle-free Vaccine Doses Ready: Report
News 18
Abiding By Centre's Supply Principle, Kids’ Vaccine ZyCoV-D To Be Supplied To Private Hospitals
News 18
National Rollout of Zydus Cadila’s Vaccine As Early as Oct 15, Says NTAGI Chief, Final Checks On
News 18
Zydus Cadila Quotes Rs 1,900 for Its Covid Vaccine Shot; Govt in Talks to Curtail Price
News 18
Merck’s Molnupiravir, Glenmark’s Nasal Spray, Zydus' Antibody Cocktail: 20+ Drugs in Pipeline for Covid
News 18
Covid Vaccine For Kids Soon? Govt to Make Zydus Cadila's 3-dose Jab Part of National Drive
News 18
Zydus Cadila Covid vaccine to be part of national vaccination programme soon: Health Ministry
India Today)
Centre working with Zydus Cadila on pricing of ZyCoV-D vaccine; likely to launch on 2 October
Firstpost
Modi Govt's Oct Bonanza: 1 Crore Doses of Zydus Cadila’s Covid Vaccine to Hit Markets Next Month
News 18
Zydus Cadila's Covid vaccine ZyCoV-D launch likely in October: Sources
India TV NewsDCGI nod to phase-I clinical trial of COVID-19 vaccine of Reliance Life Sciences
The Hindu
'ZyCoV-D Will be as Affordable as Our Other Products, Market Launch Likely by Sept-end': Zydus Group MD
News 18
Recruitment of Volunteers for Trial of Covid Vaccine Covovax on Children Begins
News 18ZyCoV-D vaccine safe and effective for adolescents, says expert
The Hindu
NTAGI to Meet This Week to Introduce Zycov-D in Covid-19 Immunisation Plan
News 18
Zydus Cadila to start making 1 crore doses of Covid vaccine by October
India Today
Zydus Cadila Becomes 6th Weapon in India's Armour Against Covid
News 18Zycov-D, India’s first COVID-19 vaccine for those above 12, gets nod
The Hindu
Zydus Cadila's 3-dose Jab Approved; India Gets its First Vaccine for Children 12 Years and Above
News 18
ZyCoV-D, World's 1st DNA Plasmid Covid-19 Vaccine Gets Approval: All About India's 2nd Homegrown Jab
News 18
Zydus Cadila's 3-dose Covid Vaccine ZyCov-D Gets Govt Panel Nod
News 18)
COVID-19 vaccines for children will arrive 'very soon', says Mansukh Mandaviya
Firstpost
Zydus Cadila's Covid vaccine gets emergency use nod, will be first jab for 12-18 year olds
India Today
When will Covid vaccines be available for children? Health Minister responds
India TV News
COVID-19 vaccines for children may be available by September: NIV director
India TV News
Covid Vaccine Tracker: J&J Doses Expected to Arrive India in Nov-Dec, Zydus Cadila Next
News 18
Zydus Cadila's Covid vaccine to get Govt's approval this week: Report
India TV News
Zydus Cadila vaccine may get nod this week, will be first jab for 12-18 year olds
India Today
Zydus Cadila to Submit Additional Data on Covid Vaccine to DGCI This Week: Sources
News 18
Covid-19 vaccine trial for children: Second dose to kids aged 2-6 years to be given next week
India Today
Zydus Cadila’s Covid vaccines for children may be available soon: Govt to court
Live Mint
Zydus Cadila's Covid vaccine for 12-18 year olds soon, clinical trials done: Centre to Delhi HC
India Today)
DCGI Recommendation On Zydus Vaccine Likely Soon, Will Allow Doses for Kids if Found Safe: Govt
News 18
Decision on Zydus needle-free Covid vaccine in few days. What you need to know
Live Mint)
Can Launch Vaccine Within 2 Months If Approved, Says Zydus Cadila on World's 1st Plasmid DNA Shot
News 18)
As Zydus Cadila Seeks Nod for World’s Ist Plasmid DNA Shot, Here’s Everything About its Science
News 18
Zydus Cadila seeks Emergency Use Authorisation for its Covid-19 vaccine ZyCoV-D in India
India TV News
Zydus Cadila seeks emergency use nod for covid vaccine, also tested in 12-18 year-olds
Live Mint
'Needle-free' Zydus Cadila vaccine to come with an applicator. Company to roll out 5 crore doses by year-end
India TodayDiscover Related