WHO Reviewing Data on Bharat Biotech’s Covaxin, Decision on Approval in '24 Hours or So'
News 18The World Health Organization was reviewing data on Covaxin, India’s indigenously-made vaccine against Covid-19 and will announce a decision on its Emergency Use Listing in “24 hours or so” a spokesperson said. WHO Chief Scientist Soumya Swaminathan had said in a tweet earlier that the technical advisory group at the organisation will meet on October 26 to consider the much-coveted emergency use listing for Covaxin being manufactured by Bharat Biotech. We are aware that many people are waiting for WHO’s recommendation for Covaxin to be included in the #COVID19 Emergency Use Listing, but we cannot cut corners – before recommending a product for emergency use, we must evaluate it thoroughly to make sure it is safe and effective, WHO had said. Ryan said we have to be absolutely sure and it’s really important that we gather all of the necessary information not only on the vaccine itself but on the manufacturing processes and all of that, because we’re recommending to the world that this vaccine is safe, effective and it’s been produced using the highest quality standards.” Further explaining how the WHO technical advisory group works, he said the vaccine manufacturers first of all have to request and respond and say that they want their vaccines to be put for EUL and then provide documentation on the whole process — the efficacy studies and the manufacturing process.
History of this topic
ICMR got ₹172 cr. from Bharat Biotech for Covaxin: Nadda
The Hindu
Bharat Biotech corrects Covaxin patent to include ICMR, NIV after govt objections: Health Minister
New Indian Express
The right way to counter a poor Covaxin safety study
The Hindu
Patent filings credit Bharat Biotech as ‘inventor’ of Covaxin, omit ICMR
The Hindu
1 in 3 Covaxin takers reported adverse events, BHU study claims
Hindustan Times
BHU study: Over 30 per cent of Covaxin recipients report adverse events
India TV News
AstraZeneca's Covishield Showed Lowest Incidence of Side Effects in India Versus Globally, Says Top Govt Official
News 18
After AstraZeneca covid-19 vax recall worldwide, Serum says it stopped manufacturing jabs in Dec 2021
Live Mint
‘Safety is primary focus’: Covaxin-developer amid reports on AstraZeneca vaccine
Hindustan Times
Covishield more effective than Covaxin: Study
New Indian Express
With State prod, India can be world leader in vaccines
Hindustan Times
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
India’s Winning Formula: Making Healthcare Affordable and Accessible with PPPs
News 18
India restarts Covid vaccine production as new infections soar 30% in one day
The Independent
Bharat Biotech starts dispatching nasal vaccines: Why it’s a big thing for India
Live Mint
Bharat Biotech’s nasal vaccine likely to be launched on Jan 26
Hindustan Times
Covid: 250 million Corbevax, Covaxin in stock, ready for dispatch
Hindustan Times
Biological E, Bharat Biotech Together Sitting on Stockpile of 250 Million Covid Vaccine Doses
News 18
Covid: Biological E, Bharat Biotech together holding 250 million vaccine doses
Live Mint
Biological E, Bharat Biotech have stockpile of 250 million COVID vaccine doses
India TV News
Rs 800 at Pvt Hosps, Rs 325 at Govt Centres: India's 1st Needle-free Nasal Vax, Now Available as Booster
News 18Bharat Biotech’s nasal vaccine to be available as booster dose
The Hindu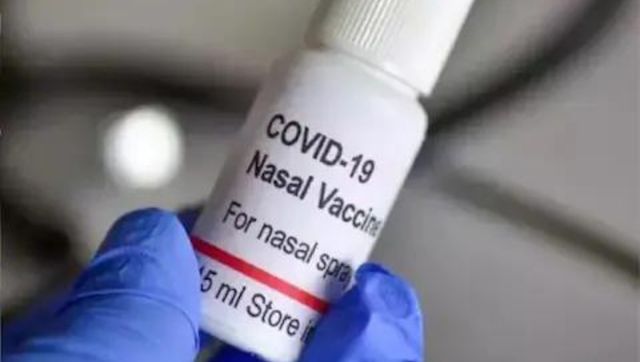)
COVID-19: Centre approves Bharat Biotech's intranasal vaccine, to be available on CoWIN
Firstpost
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News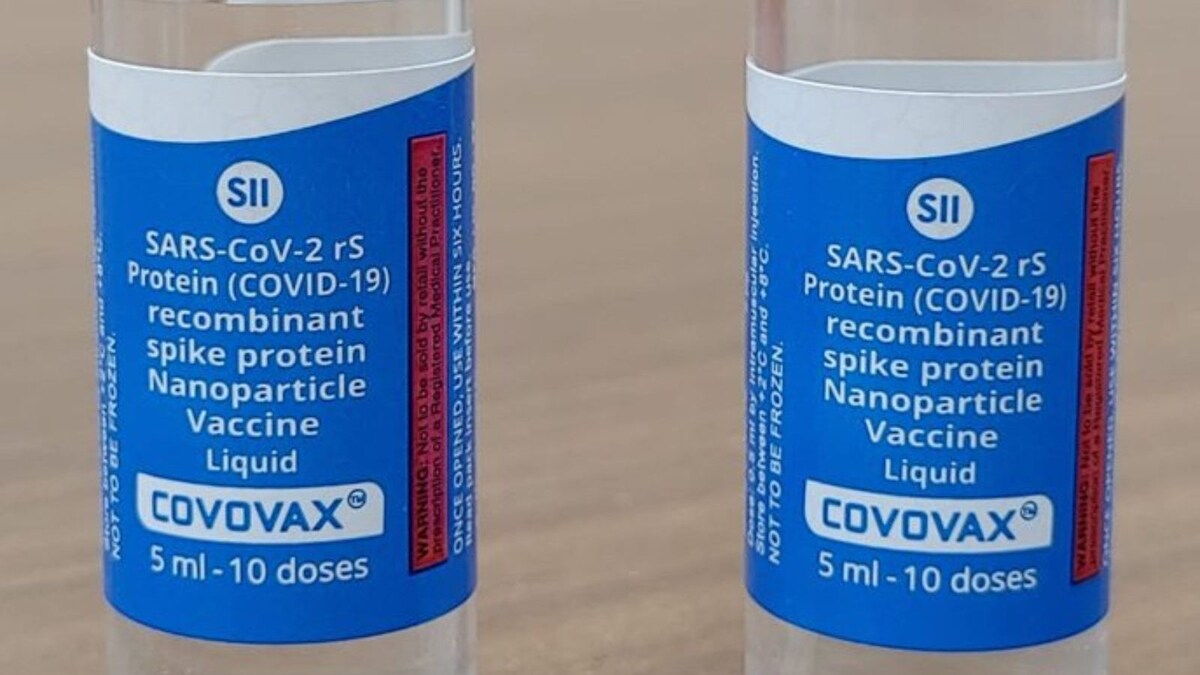
SII Seeks Drug Regulator's Nod for Market Authorisation of Covovax Vaccine as Covid-19 Booster Dose
News 18
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
Bharat Biotech misstated Covaxin trial data
Deccan Chronicle
Govt, Bharat Biotech Deny ‘Shortcut’ in Covaxin Trials: What's the Controversy?
The Quint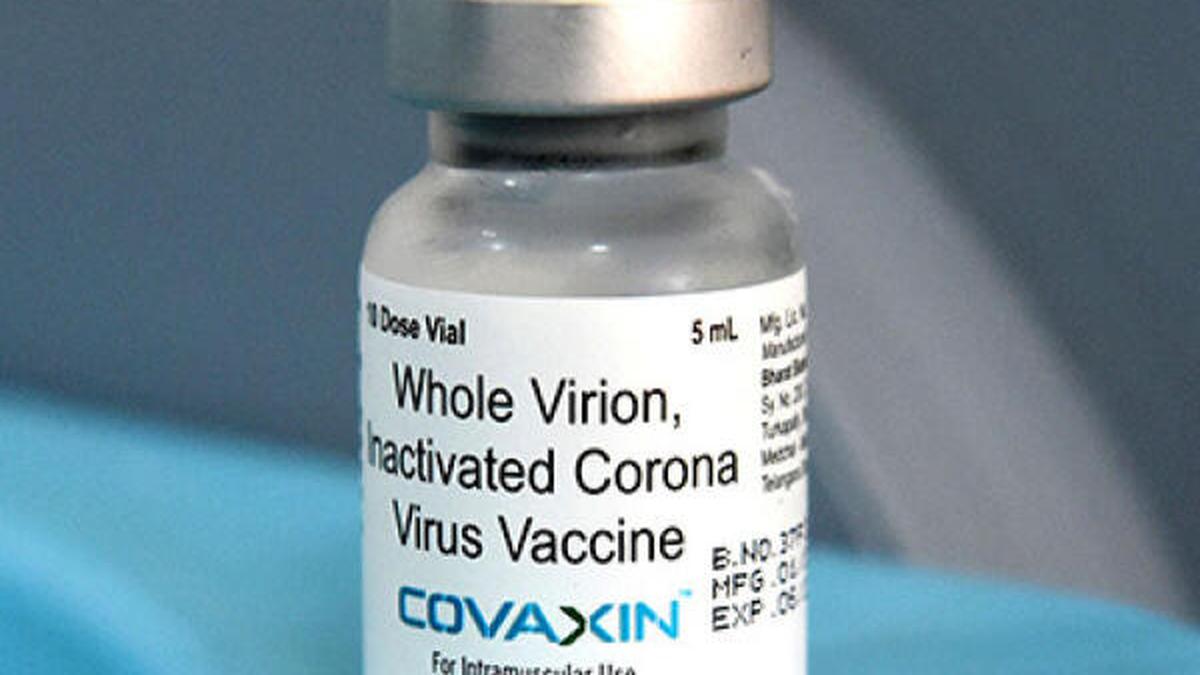
50 million doses of Covaxin set to expire early 2023 due to poor offtake
The Hindu
COVID: 50 million Covaxin doses set to expire early 2023 due to poor off take
India TV News
Corbevax approved as booster for those jabbed with Covishield, Covaxin: Report
Live Mint
In A First, Biological E's Corbevax Approved As Covid Booster for Covishield, Covaxin Recipients
News 18
Govt Nod for Corbevax as Booster for Adults Vaccinated with Covishield, Covaxin Soon
News 18
Adults may get different vaccine as booster shot instead of Covishield, Covaxin
Live Mint
India's Covid Vaccinations Hit 200 Crore Mark in Big Milestone for 7-Phase Drive Under Modi's Leadership
News 18
AstraZeneca and Pfizer Vaccines Saved Over 1.2 Crore Lives in First Year: Study
News 18
NTAGI Panel Recommends Use of Covid Vaccines Corbevax, Covaxin for Kids Aged 5-12 Years
News 18
Corbevax, Covaxin vacines get nod for use among 5-12 years olds; details here
Live Mint
Serum Institute gets govt nod to export 32.4 lakh doses of Covovax vaccine to US
India Today
Allowing Corbevax as booster for those vaccinated with Coivishield, Covaxin likely to be considered by NTAGI
The Hindu
Bharat Biotech Nasal Covid Vaccine's Phase 3 Trials Completed
News 18
Covaxin demonstrates robust safety and immunogenicity in children 2-18 years: Bharat Biotech
The Hindu)
Covaxin safe, well-tolerated in 2-18 years age-group, announces Bharat Biotech
Firstpost
Corbevax Gets DCGI Approval As Covid-19 Vaccine Booster Dose For 18 Years And Above
ABP News
Germany recognises Bharat Biotech's Covaxin for travel from June 1
India TV News
Jabs for Kids, Reducing Gap Between Doses for Those Travelling Abroad on Agenda as NTAGI Meets Today
News 18
No Haste In Granting Emergency Use Authorization To Covaxin & Covishield: Supreme Court
Live Law
SEC seeks more data from Serum Institute of India over COVID vaccine 'Covovax' for 7-12 age group: Sources
India TV News
COVID-19: Govt to expedite process of recognition of Biological E Corbevax by other countries
The Hindu
In haste: The Hindu Editorial on vaccinating children against COVID-19
The HinduDiscover Related







)