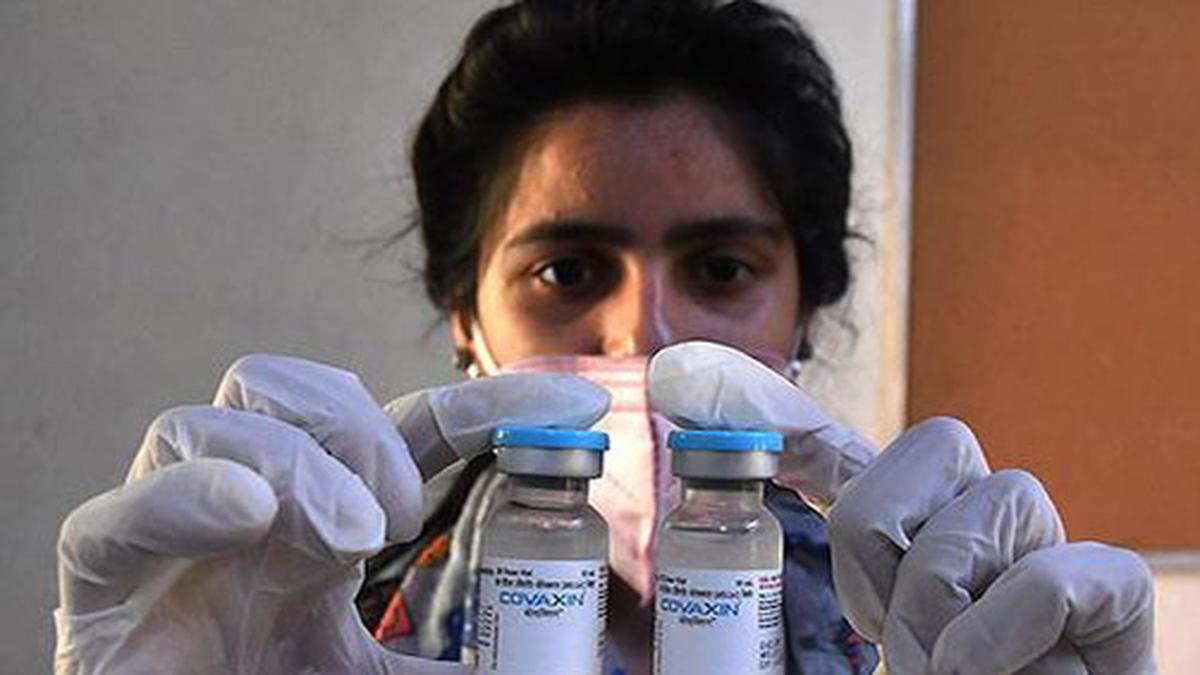
Tata group's low cost Covid-19 test 'Feluda' gets govt nod for launch
India TodayThe Tata group on Saturday received approval for the commercial launch of India's first CRISPR Covid-19 test, 'Feluda', by the Drugs Controller General of India. "The Tata CRISPR test, powered by CSIR-IGIB FELUDA, received regulatory approvals today from DCGI for commercial launch, as per ICMR guidelines, meeting high quality benchmarks with 96 per cent sensitivity and 98 per cent specificity for detecting novel coronavirus," the statement said. The Tata CRISPR test is the world's first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing COVID-19, it said. Commenting on the development, Girish Krishnamurthy, CEO, TATA Medical and Diagnostics Ltd said, "The approval for the Tata CRISPR test for COVID-19 will give a boost to the country's efforts in fighting the global pandemic. "The commercialisation of Tata CRISPR test reflects the tremendous R&D talent in the country which can collaborate to transform India's contributions to the global healthcare and scientific research world," he said.
History of this topic
An epidemiologically sound testing strategy
The Hindu
Can Genetic Modification Technology CRISPR be Applied to Covid-19 diagnostics?
News 18)
Tata's Healthcare Arm Ties Up With CSIR for Covid-19 Testing Across Smaller Towns and Rural Areas
News 18
India's first mRNA Covid vaccine candidate gets nod to start phase 1 trials
India Today
Coronavirus test: Delhi to get Feluda paper strip test kit today
Live MintCoronavirus India lockdown Day 211 updates | October 23, 2020
The HinduCoronavirus | Feluda test to be commercially available by month-end: CSIR Director General
The Hindu
Detect coronavirus in just 5 min: Nobel Prize winner develops unique Covid test
India Today)
India's Feluda Covid-19 Test Cheaper, Faster Alternative to RT-PCR, Say Scientists
News 18
Feluda Is Back! This Time To Detect Covid-19 Infection; Tata CRISPR's Test Gets DCGI Approval For Commercial Launch
ABP News)
India's First Paper-strip Covid-19 Test Named After Satyajit Ray's 'Feluda', All You Need to Know
News 18)
India surpasses US in global COVID-19 recoveries as tally exceeds 53 lakh; SII to begin phase III trials from Monday
Firstpost)
Drug Body Approves Commercial Launch of Low Cost Covid-19 Test 'Feluda'
News 18
Tata group to launch India's first CRISPR coronavirus test 'Feluda'
India TV News)
India's First 'Tata CRISPR' Covid-19 Test Gets Approval by DCGI for Commercial Launch
News 18
India, Israel developing covid-19 rapid testing kits: Commander Yaniv Meirman
Live Mint)
COVID-19 vaccine in India: Government to set guidance document for trials of vaccines, treatment
FirstpostDiscover Related