)
WHO decision on Bharat Biotech's Covaxin emergency use listing likely next week
FirstpostThe Strategic Advisory Group of Experts on Immunization of the WHO met on Tuesday to make its recommendations on Covaxin on EUL, among other issues New Delhi: The WHO will take a call on granting Emergency Use Listing status for Bharat Biotech’s COVID-19 vaccine Covaxin next week, the global health body said on Tuesday. “WHO and an independent group of experts are scheduled to meet next week to carry out the risk/benefit assessment and come to a final decision whether to grant Emergency Use Listing to Covaxin,” the global health body tweeted. Bharat Biotech gave a presentation on the Vaccine’s safety and efficacy data of clinical trials and Risk management plans and other implementation considerations, according to the SAGE draft agenda. The indigenously developed Bharat Biotech’s Covaxin is one of the six vaccines that have received emergency use authorisation from India’s Drug Regulator and is being used in the nationwide anti-COVID-19 inoculation programme along with Covishield and Sputnik V.
History of this topic
ICMR got ₹172 cr. from Bharat Biotech for Covaxin: Nadda
The Hindu
The right way to counter a poor Covaxin safety study
The Hindu
1 in 3 Covaxin takers reported adverse events, BHU study claims
Hindustan Times
BHU study: Over 30 per cent of Covaxin recipients report adverse events
India TV News
‘Safety is primary focus’: Covaxin-developer amid reports on AstraZeneca vaccine
Hindustan Times
With State prod, India can be world leader in vaccines
Hindustan Times
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
Serum institute seeks to add Covovax on CoWIN portal as booster dose for adults
Hindustan Times
DCGI Approves Market Authorisation for SII's Covid Vaccine Covovax as Heterologous Booster Dose
News 18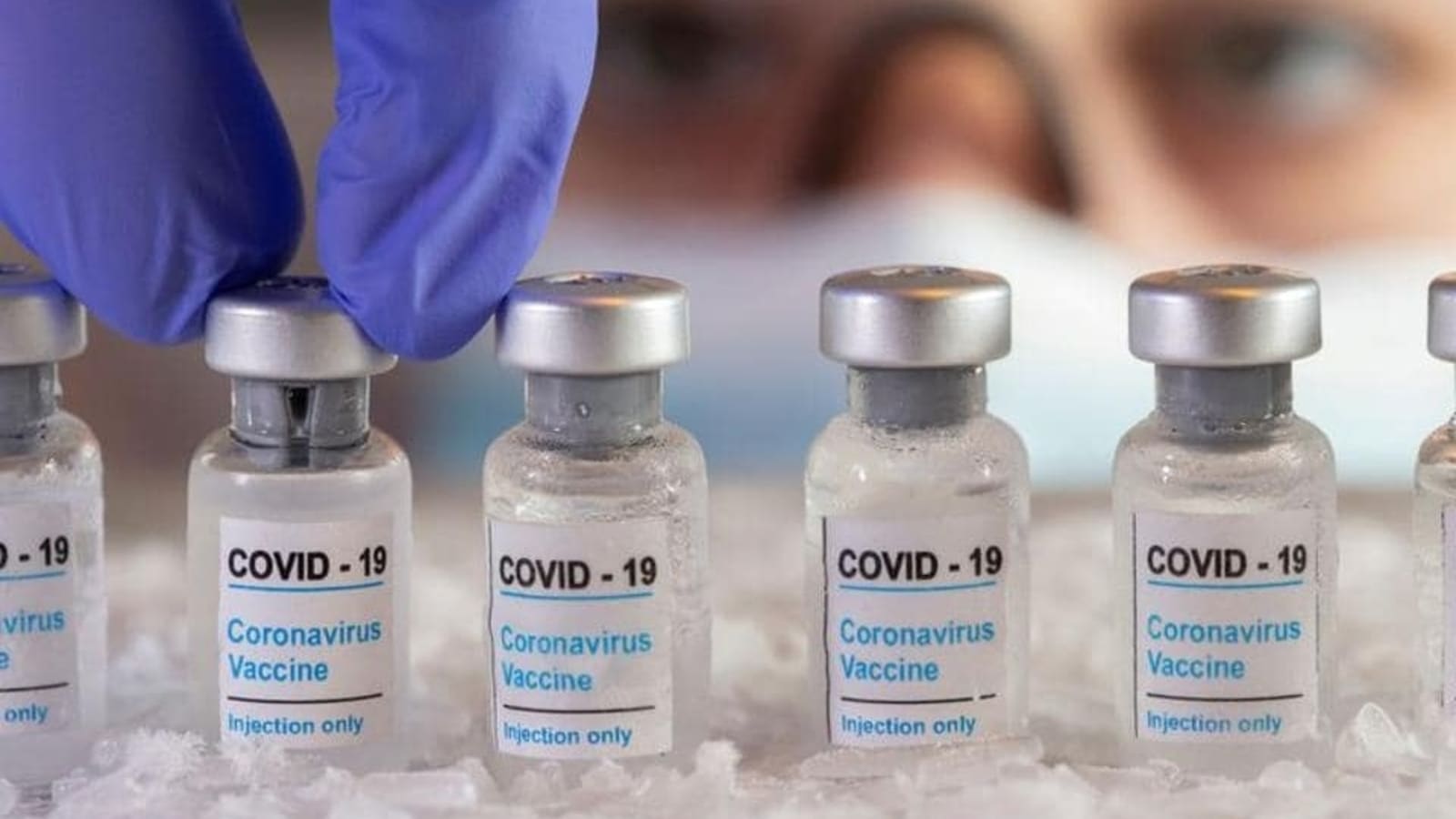
Covid: 250 million Corbevax, Covaxin in stock, ready for dispatch
Hindustan Times
Biological E, Bharat Biotech Together Sitting on Stockpile of 250 Million Covid Vaccine Doses
News 18
Covid: Biological E, Bharat Biotech together holding 250 million vaccine doses
Live Mint
Biological E, Bharat Biotech have stockpile of 250 million COVID vaccine doses
India TV News
Rs 800 at Pvt Hosps, Rs 325 at Govt Centres: India's 1st Needle-free Nasal Vax, Now Available as Booster
News 18
Nasal vaccines to roll out in private hospitals soon
Hindustan Times
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News
SII Seeks Drug Regulator's Nod for Market Authorisation of Covovax Vaccine as Covid-19 Booster Dose
News 18
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
Bharat Biotech misstated Covaxin trial data
Deccan Chronicle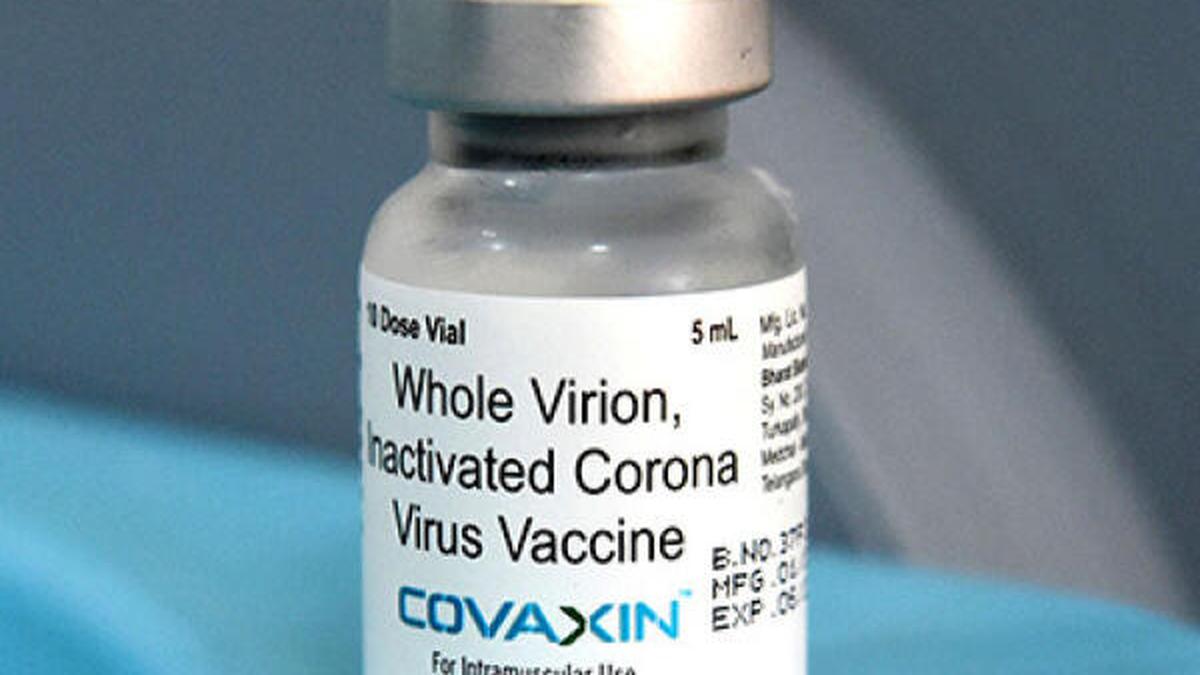
50 million doses of Covaxin set to expire early 2023 due to poor offtake
The Hindu
COVID: 50 million Covaxin doses set to expire early 2023 due to poor off take
India TV News
COVID-19: Corbevax to be given as boosters at vaccination centres from today
Live Mint
Corbevax as Covid booster for adults from August 12. Check details
India Today
Corbevax approved as booster for those jabbed with Covishield, Covaxin: Report
Live Mint
In A First, Biological E's Corbevax Approved As Covid Booster for Covishield, Covaxin Recipients
News 18
Corbevax approved as precaution dose for adults vaccinated with Covaxin, Covishield
India TV News
Govt Nod for Corbevax as Booster for Adults Vaccinated with Covishield, Covaxin Soon
News 18
Adults may get different vaccine as booster shot instead of Covishield, Covaxin
Live Mint
NTAGI Panel Recommends Use of Covid Vaccines Corbevax, Covaxin for Kids Aged 5-12 Years
News 18
Corbevax, Covaxin vacines get nod for use among 5-12 years olds; details here
Live Mint
Serum Institute gets govt nod to export 32.4 lakh doses of Covovax vaccine to US
India Today
Allowing Corbevax as booster for those vaccinated with Coivishield, Covaxin likely to be considered by NTAGI
The Hindu
Covaxin demonstrates robust safety and immunogenicity in children 2-18 years: Bharat Biotech
The Hindu)
Covaxin safe, well-tolerated in 2-18 years age-group, announces Bharat Biotech
Firstpost
Covaxin Effective Against Omicron Variants, Says ICMR as Sub-variants Driving up US Surge Found in Maha, Tamil Nadu
News 18
Corbevax Gets DCGI Approval As Covid-19 Vaccine Booster Dose For 18 Years And Above
ABP News
Germany recognises Bharat Biotech's Covaxin for travel from June 1
India TV News
SII's Covid Vaccine Covovax Now Available for 12-17 Age Group at Private Centres
News 18
No Haste In Granting Emergency Use Authorization To Covaxin & Covishield: Supreme Court
Live Law
SEC seeks more data from Serum Institute of India over COVID vaccine 'Covovax' for 7-12 age group: Sources
India TV News
COVID-19: Govt to expedite process of recognition of Biological E Corbevax by other countries
The Hindu
In haste: The Hindu Editorial on vaccinating children against COVID-19
The Hindu
COVID: Over 2,900 new pandemic cases, 32 fatalities in last 24 hours; active cases rise to 16,279
India TV News
Covaxin Gets DCGI Nod for Kids Aged 6-12 Yrs; Corbevax Cleared for 5-12 Yr Olds; 12+ to Get Zydus Jab
News 18
Vaccine for kids: DCGI grants emergency use nod to 3 Covid vaccines for different age group of children
India TV News
India Could Start Vaccinating Younger Kids Soon as Govt Panel Suggests Covaxin for 6-12 Age Group - News18
News 18
Bharat Biotech asked to provide more data on Covaxin for below 12-year-olds
Deccan Chronicle
Govt panel approves use of Corbevax for 5-12 year age group
India Today
Morning Digest | Four militants behind J&K targeted killings shot dead; Covaxin trials in U.S. put on hold, and more
The Hindu
Weighing the choices: on precautionary vaccine doses
The HinduDiscover Related