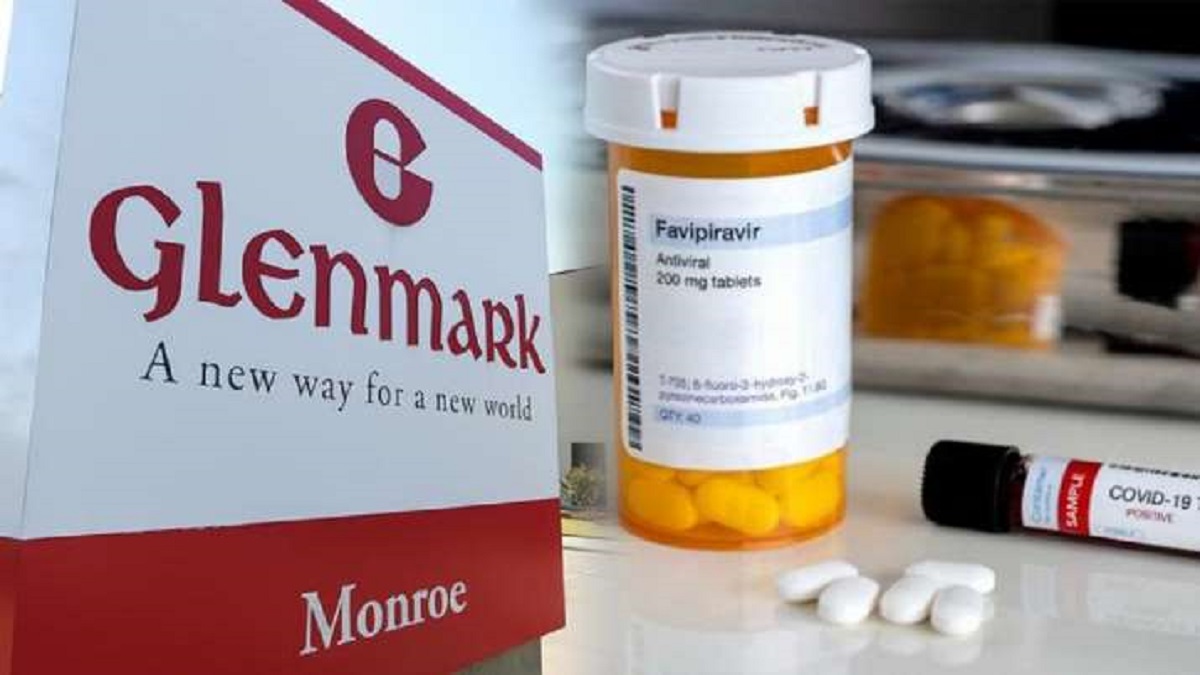
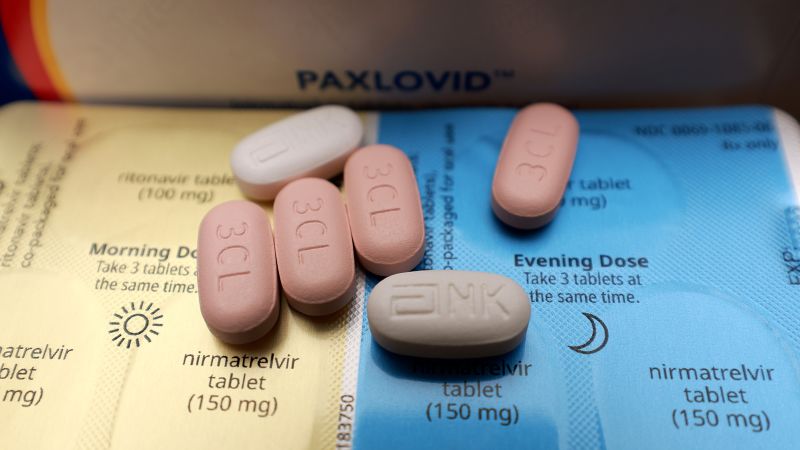
DCGI sends notice to Glenmark for false claims, overpricing of COVID-19 drug FabiFlu
India TV NewsThe Drug Controller General of India has sent a show cause notice to Glenmark Pharmaceuticals over 'overpricing' and alleged 'false claims' of its drug Fabiflu. The DCGI also said that Glenmark made claims that Fabiflu alone would suffice as treatment in mild and moderate coronavirus cases and that it is effective for patients with comorbidities. "It has been mentioned in representation that Glenmark has claimed that this drug is effective in co-morbid conditions like hypertension, diabetes whereas in reality, as per protocol summary, this trial was not designed to assess the Fabiflu in co-morbid conditions. The notice stated that the DCGI has received reports that Fabiflu -- a version of favipiravir -- has been priced at Rs 12,500 for the entire course.
History of this topic

Prices of 800 essential medicines, including Paracetamol, set to rise by 10.7% from April
India Today)
Paracetamol, Azithromycin, Ivermectin: Essential Medicines Might Face Shortage If API Costs Continue to Rise
News 18)
BDR Pharma To Launch Favipiravir Tablets for Treating Covid-19 Patients at Rs 990
News 18)
Brinton Pharma Gets DCGI Nod to Launch Favipiravir 400 mg Tablets for Covid-19 Treatment
News 18)
Glenmark to Launch 400 mg of Antiviral Drug FabiFlu for Covid-19 Treatment
News 18)
Zenara Pharma gets DCGI approval to sell Favipiravir tablet Favizen to treat mild to moderate COVID-19 infections
Firstpost)
Zenara Pharma Receives DCGI Approval to Manufacture, Sell Favipiravir Tablets
News 18)
Lupin launches antiviral drug Favipiravir under the name Covihalt to treat mild to moderate COVID-19 infection
Firstpost)
Sun Pharma Launches Antiviral Covid-19 Drug Favipiravir in India at Rs 35 Per Tablet
News 18The many questions about Favipiravir
The Hindu
Glenmark says FabiFlu reduces viral load by 4 days, treats mild to moderate COVID-19 patients
India TV News
Glenmark Pharmaceuticals shares soar 20 per cent after COVID-19 drug Favipiravir launch
India TV NewsHetero launches drug for COVID-19 patients
The HinduCoronavirus | Drug launched for moderate COVID-19 cases
The HinduCoronavirus | Glenmark’s antiviral drug Fabiflu to be used for patients with mild and moderate symptoms
The Hindu
Glenmark launches COVID-19 drug after DCGI nod
India TV News)
Indian regulators give Glenmark approval to manufacture, sell favipiravir for ‘restricted emergency use’ in treating COVID-19 patients
FirstpostDiscover Related




























)