
India finds serious breaches, halts production over Gambia deaths
Al JazeeraWHO report found the Maiden Pharmaceuticals medicine may be linked to the deaths of dozens of children in the African nation. Indian health authorities said on Wednesday they had halted all production of New Delhi-based Maiden Pharmaceuticals after a WHO report that its cough and cold syrups exported to The Gambia may be linked to the deaths of dozens of children there. The UN health agency said that laboratory analysis of four Maiden products – Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup – had “unacceptable” amounts of diethylene glycol and ethylene glycol, which can be toxic and lead to acute kidney injury. Gambian police, in a preliminary investigation report on Tuesday, said that the deaths of 69 children from acute kidney injury were linked to the cough syrups made in India and imported via Atlanta-based Atlantic Pharmaceuticals. The firm is distinct from Atlanta-based Atlantic Pharmaceuticals, Inc, whose president Anthony Soscia told Reuters by email that his company “does not import or export any pharmaceuticals.” The cough syrups had been approved for export only to The Gambia, India said, although the WHO says they may have gone elsewhere through informal markets.
History of this topic

How is India streamlining the pharma sector? | Explained
The Hindu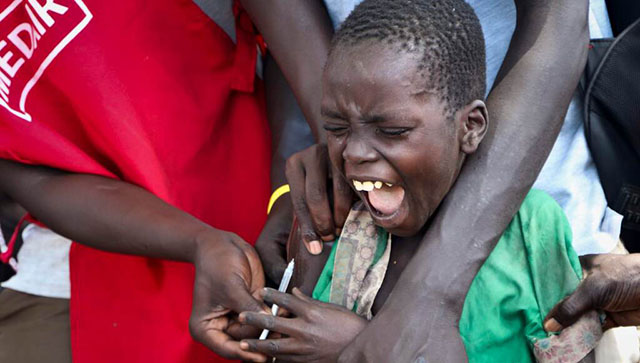)
Vantage | Why poor quality cough syrups may poison India's reputation too
Firstpost
How India’s Vaccine Industry Will Save Millions of Children in Africa - News18
News 18)
India finds 2 more toxic cough syrups: What we know about them
Firstpost
Why does India have a substandard drugs problem? | In Focus podcast
The HinduExplained | Why are Indian drugmakers under the lens?
The Hindu
'India will never bargain on quality of medicines': Health minister Mandaviya on cough syrup row
Hindustan Times
No tolerance on spurious meds, 71 firms issued notices: Mandaviya on cough syrup row
Deccan Chronicle
Safety first: on Indian pharma products and drug safety
The Hindu
India's Claim to ‘Pharmacy of the World’ in Limbo, Fixing Quality of Drugs an Emergency - News18
News 18
Test cough syrups meant for exports on priority: DCGI
Hindustan Times
India considers policy change after cough syrup deaths: PM Modi's office
The Hindu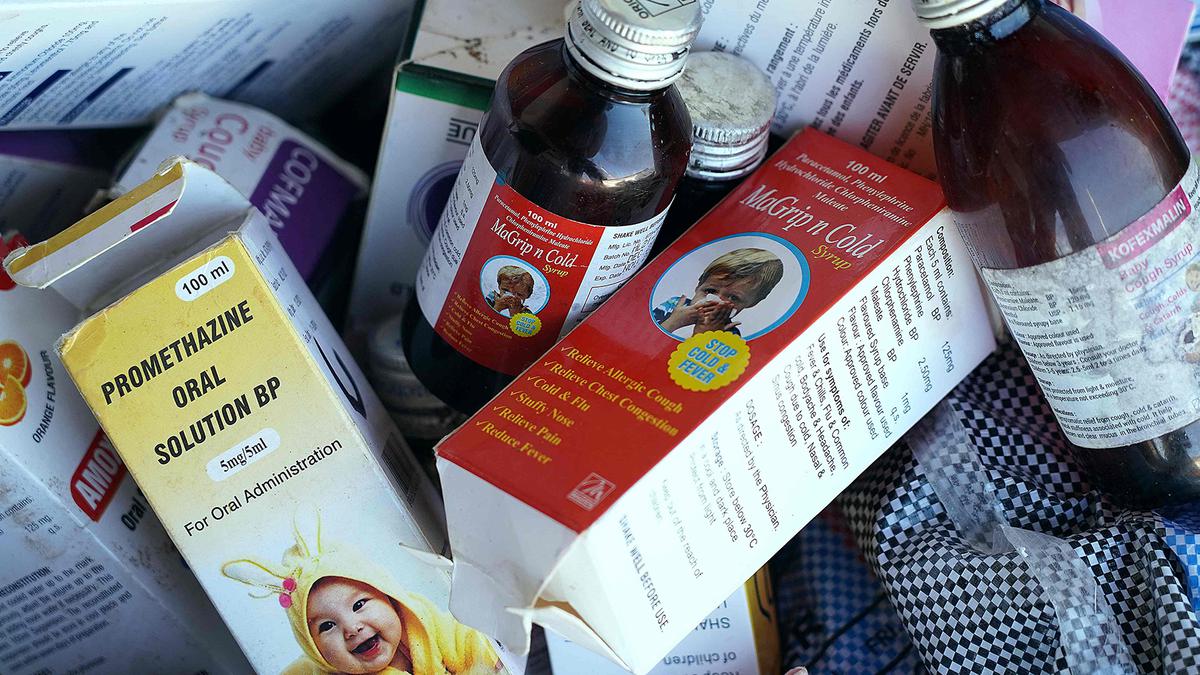
Indian cough syrup: mystery middleman may be new clue
The Hindu
Indian cough syrup: Mystery middleman may be new clue
Live Mint)
WHO issues alert for another India-made cough syrup: Why are these drugs so deadly?
Firstpost
Guaifenesin: WHO flags India-made ‘contaminated’ cough syrup with ‘unacceptable amounts’ of toxic chemicals
The IndependentContaminated syrups manufactured in India reported in WHO’s Western Pacific region
The Hindu
Cough Syrup Deaths in Gambia, Uzbekistan: 18 Indian Drug Makers Lose Licence in Big Clean-Up
News 18
Licences of six cough syrup makers in Maharashtra suspended for violation of rules
The Hindu
Cough Syrup Death Cases: US CDC Blames Indian Pharma Firm for Demise of Gambia Kids; 3 Held in Noida Over Uzbekistan Incident
News 18
U.S.-CDC probe into cough syrup deaths in The Gambia pins blame on Indian manufacturer
The Hindu
Cough Syrup Row: Maharashtra Govt Acted against 27 Pharma Firms, Says Minister
News 18
Gambia, Uzbekistan Effect? Modi Govt Plans Two-day 'Chintan Shivir' of Drug Regulators, Policymakers
News 18WHO calls for action to protect children from contaminated medicines after cough syrups deaths
The Hindu
Cough Syrup deaths: WHO investigating links between manufacturers in India
Live Mint
WHO calls for action to protect children from contaminated medicines after cough syrups deaths
India TV News
WHO Urges 'Immediate and Coordinated Action' on Contaminated Medicines After Child Deaths
News 18
Indonesia court hearing into children killed by toxic cough syrup
Al Jazeera
Uzbekistan, Gambia Cough Syrup Cases: 'Concerned' Commerce Ministry Advises Thorough Probe - News18
News 18
Behind the Indian cough syrup that killed many in Gambia and Uzbekistan
The Hindu
'Substandard...': WHO warns against use of cough syrups linked to Uzbek deaths
Hindustan Times
India cough syrup linked to Uzbekistan deaths ‘substandard’: WHO
Al Jazeera)
Africa needs reliable and resilient healthcare system: How India can deliver it
Firstpost
At the precipice of shame: The Hindu Editorial on The Gambia and Uzbekistan cough syrup deaths
The Hindu
BJP, Cong spar over cough syrup related deaths in Uzbekistan, Gambia
Deccan Chronicle
India seeks details of Uzbekistan’s investigation into cough syrup
Hindustan Times
Congress, BJP trade barbs over cough syrup-related deaths of children in Uzbekistan and Gambia
The Hindu
Cough syrup deaths | India seeks details from Uzbekistan on investigations
The Hindu
Indian pharma under scrutiny again after 18 Uzbek child deaths
Al Jazeera
18 children dead due to cough syrup made by Indian firm, says Uzbekistan
The Hindu
Uzbekistan Investigates Deaths of 18 Children 'After Consuming India-made Syrup', WHO Assisting Probe
News 18
Uzbekistan Investigates Deaths of 18 Children 'After Consuming India-made Syrup', WHO Assisting Probe
News 18)
WHO drew premature link between children deaths in Gambia, India-made cough syrups: DCGI
Firstpost
WHO drew premature link between Gambia children deaths, India-made cough syrups: DCGI
Deccan Chronicle
Samples Not Contaminated, Meets Standards: Centre Slams WHO Over Premature Links Between Children Deaths in Gambia and India-made Syrups
News 18
Indian-made cough syrups did not cause Gambia children deaths, DCGI informs WHO
India TV News
'Compliant with norms': India to WHO on cough syrups and Gambia kids' deaths
Hindustan Times)
Gambia children deaths: Maiden Pharma's cough syrup samples found to be of standard quality, says govt
Firstpost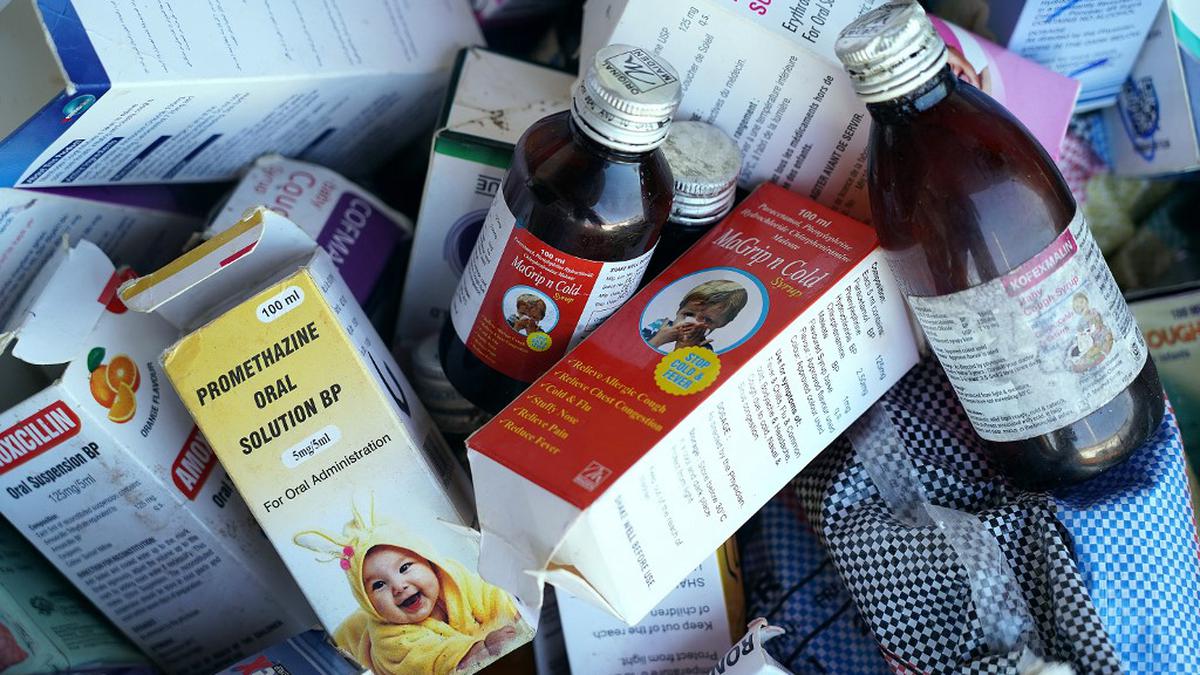
The Gambia children deaths | WHO drew premature link with India-made cough syrups, says drugs regulator
The Hindu
‘Too late to save him’: Indonesian children killed by cough syrup
Al JazeeraDiscover Related





)








)




)

















)




