After UK, Pfizer seeks emergency use approval of Covid vaccine in India: Report
India TodayDays after its parent company secured clearance in the UK and Bahrain for mass use, Pfizer India has become the first pharmaceutical firm to seek from the Drugs Controller General of India an emergency use authorisation for its Covid-19 vaccine in the country. "Pfizer India has submitted an application on December 4 to the DCGI seeking Emergency Use Authorization for its COVID-19 vaccine in India," a source told PTI. Minus 70 degrees Celsius storage The extreme low temperature of minus 70 degrees Celsius required for storing the Pfizer vaccine poses a big challenge for its delivery in a country like India, especially in its smaller towns and rural areas where maintaining such cold chain facilities would be very difficult, top government officials have said. Dr Reddy's Laboratories and the Russian Direct Investment Fund have announced that they commenced adaptive phase 2 and 3 clinical trials for Covid-19 vaccine Sputnik V in India, Also, Biological E. Ltd has started early phase 1 and 2 human trials of its Covid-19 vaccine candidate.
History of this topic
Serum Institute 'hopeful' of producing Mpox vaccine, says CEO Adar Poonawalla
India TV News
After AstraZeneca covid-19 vax recall worldwide, Serum says it stopped manufacturing jabs in Dec 2021
Live Mint
India eyes to increase oral polio vaccine supply
Live Mint
With State prod, India can be world leader in vaccines
Hindustan Times
COVID-19 Update: India records 260 new cases, total active at 1,828
Live Mint
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
India urges G-20 countries to join vaccine research collaborative in wake of COVID vaccine inequity
The Hindu
Explained | What are the problems with India’s clinical trials registry?
The HinduIndia sees over 7,800 COVID cases in a day reported on April 12, 2023
The Hindu)
‘The Vial’ trailer out: Manoj Bajpayee narrates History TV18's documentary on India’s Covid-19 vaccine journey
Firstpost
India records over 300 new Covid cases after 97 days
Hindustan Times
Covid vaccine mission is a lesson for the world
Hindustan Times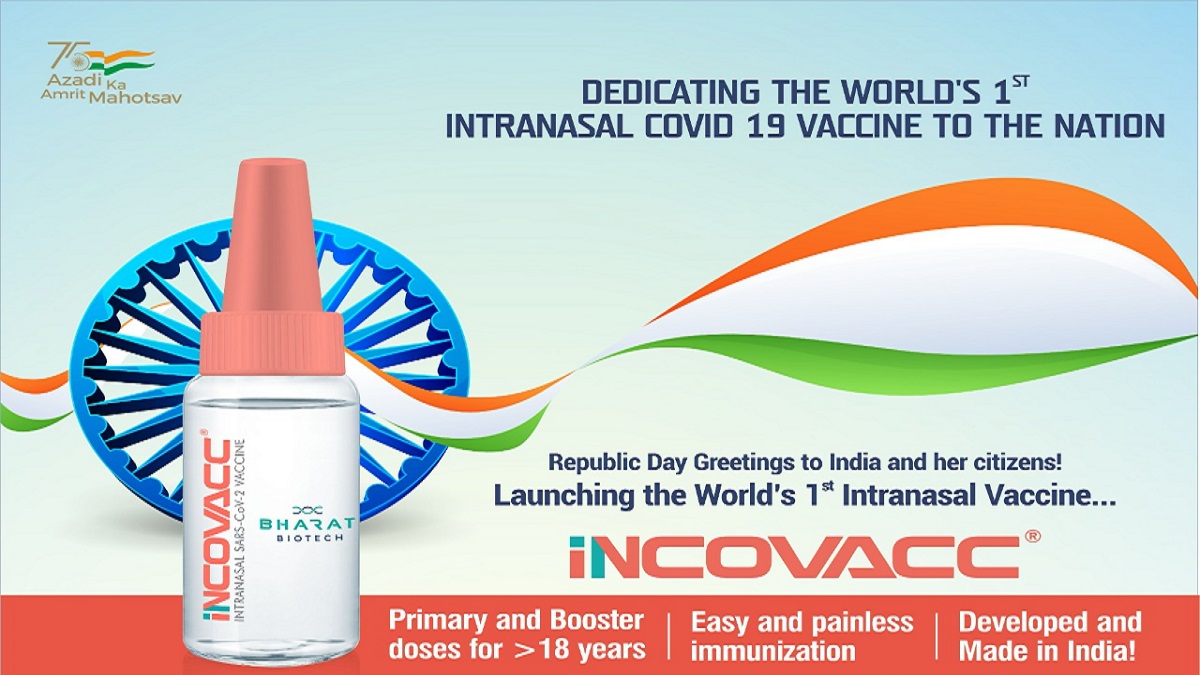
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News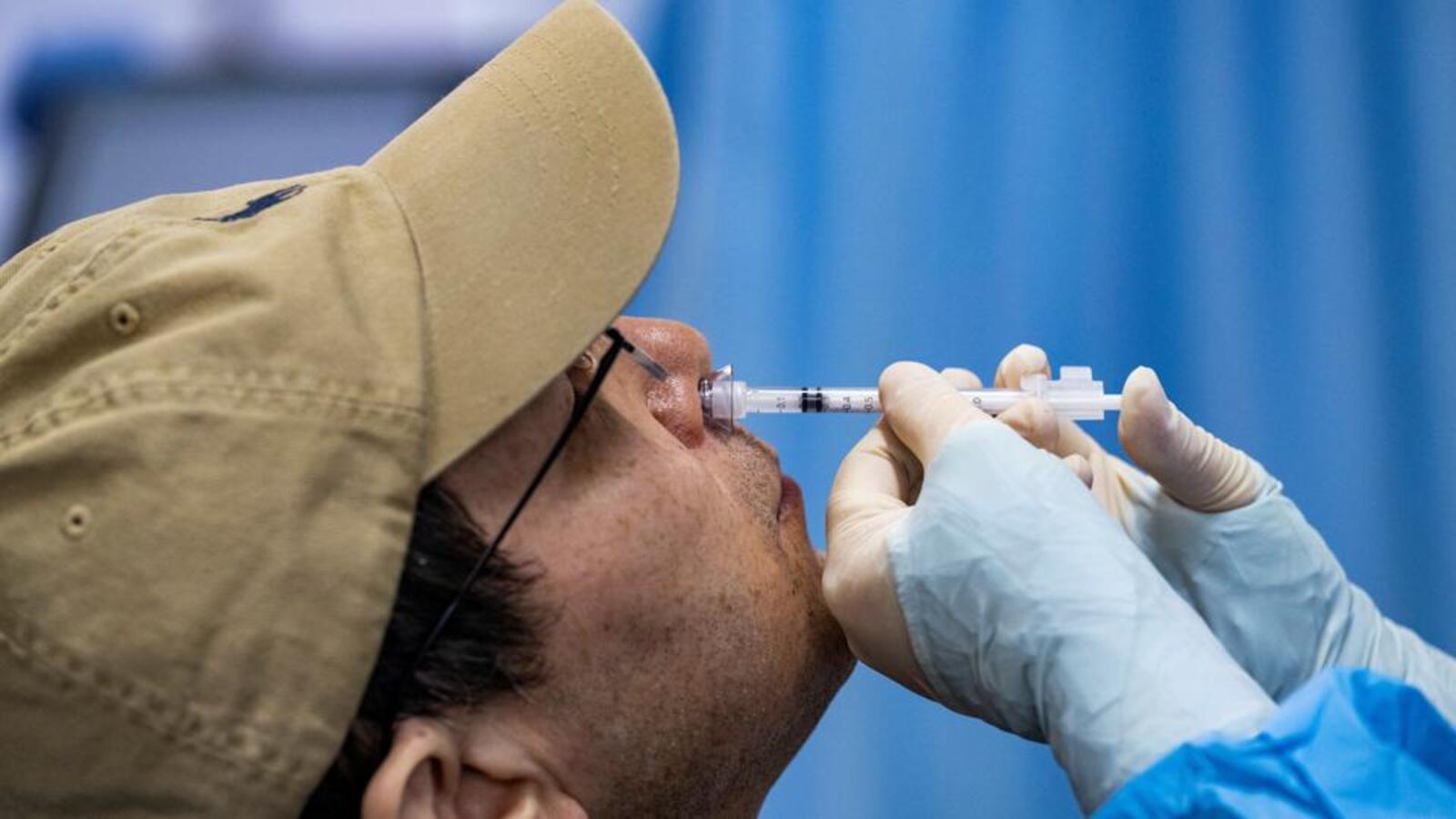
Nasal vaccine at govt centres in Delhi from Feb 15
Hindustan Times
Centre rolls out first nasal Covid vaccine as booster
Hindustan Times
Health minister Mandaviya launches Bharat Biotech's nasal Covid vaccine
Hindustan Times
Private hospitals with advance orders to get intranasal COVID-19 shots soon
The Hindu
COVID-19: Mansukh Mandaviya launches world's 1st Made-in-India nasal vaccine iNCOVACC
India TV News
Bharat Biotech’s nasal vaccine likely to be launched on Jan 26
Hindustan Times
First Indian intranasal Covid vaccine by Bharat Biotech to be launched on Jan 26
India TV News
Pfizer CEO Cornered at Davos, India Saw Through Pharma Giant’s Promises Early to Not Bank on it
News 18
DCGI Approves Market Authorisation for SII's Covid Vaccine Covovax as Heterologous Booster Dose
News 18
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The Hindu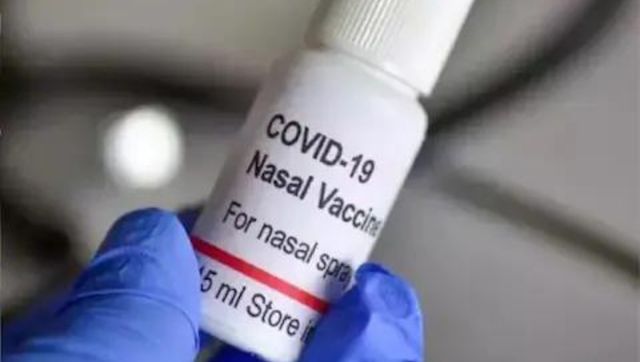)
COVID-19: Centre approves Bharat Biotech's intranasal vaccine, to be available on CoWIN
Firstpost
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News
SII Seeks Drug Regulator's Nod for Market Authorisation of Covovax Vaccine as Covid-19 Booster Dose
News 18India need not panic given excellent COVID-19 vaccination coverage: Adar Poonawalla
The Hindu
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
No Wastage of Covid Vaccines in Govt Buffer Stock Due to Expiry: Govt in Lok Sabha
News 18
World’s first intra-nasal vaccine for COVID gets CDSCO nod for restricted use
Live Mint
What Makes India's Covid Vaccination Programme A Global Best Practice In Public Health
News 18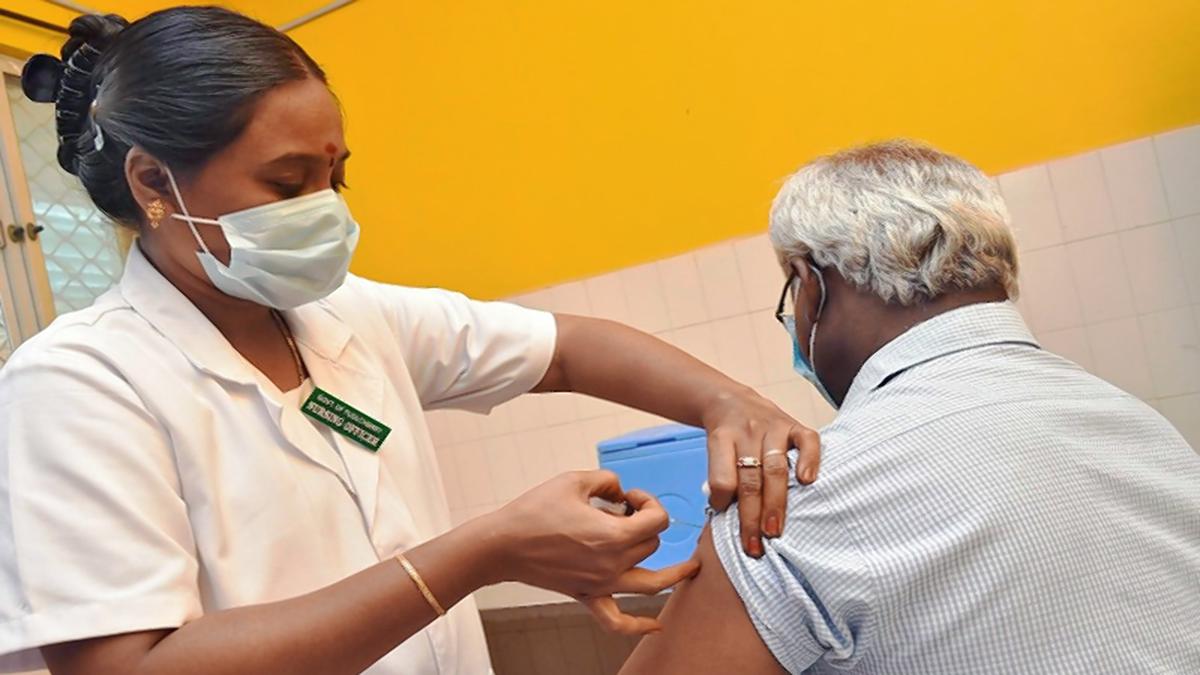
Health Ministry not to procure fresh COVID-19 vaccines; surrenders ₹4,237 crore from vaccination budget
The Hindu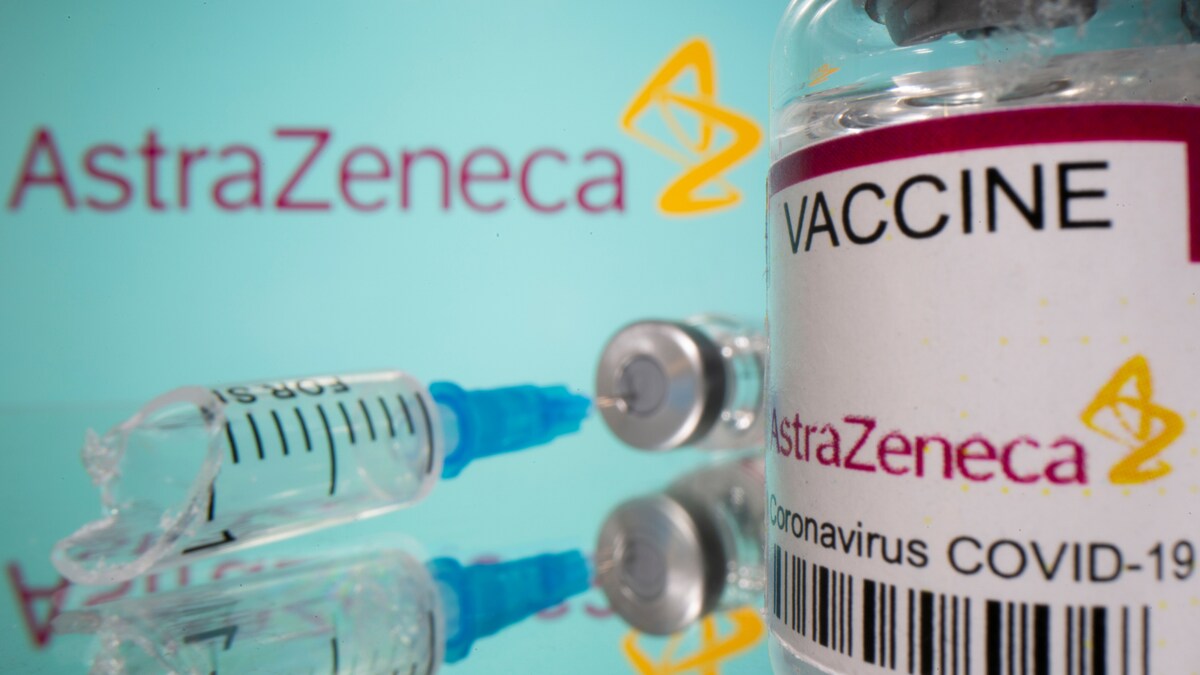
AstraZeneca Nasal Spray Vaccine for Covid-19 Suffers Setback in Early Trial
News 18
Covid-19: India logs nearly 5,000 new infections, active cases drop below 45k
Live Mint
Will Two ‘Made-in-India’ Vaccines Be the New Covid Boosters? News18 Decodes Differences
News 18
Bharat Biotech’s intra-nasal COVID vaccine gets emergency use approval
The Hindu
India's First Nasal Vaccine for Covid-19, Made by Bharat Biotech, Cleared for Emergency Use
News 18
Covid-19: India's first intranasal vaccine by Bharat Biotech gets DCGI approval
India TV News
Monkeypox vaccines: India puts emergency buying on hold for now - here’s why
Live Mint
An End to Foreign-return Vaccine Woes: India to Offer Remaining Covid-19 Shots Domestically
News 18
Tackling the new variants
Hindustan Times
COVID-19 intranasal vaccine Phase III trials over, proven safe, says Bharat Biotech
Deccan Chronicle
COVID-19: Corbevax to be given as boosters at vaccination centres from today
Live Mint
Corbevax Likely to be Available As Covid Booster Dose at Vaccination Centres from Friday
News 18
In A First, Biological E's Corbevax Approved As Covid Booster for Covishield, Covaxin Recipients
News 18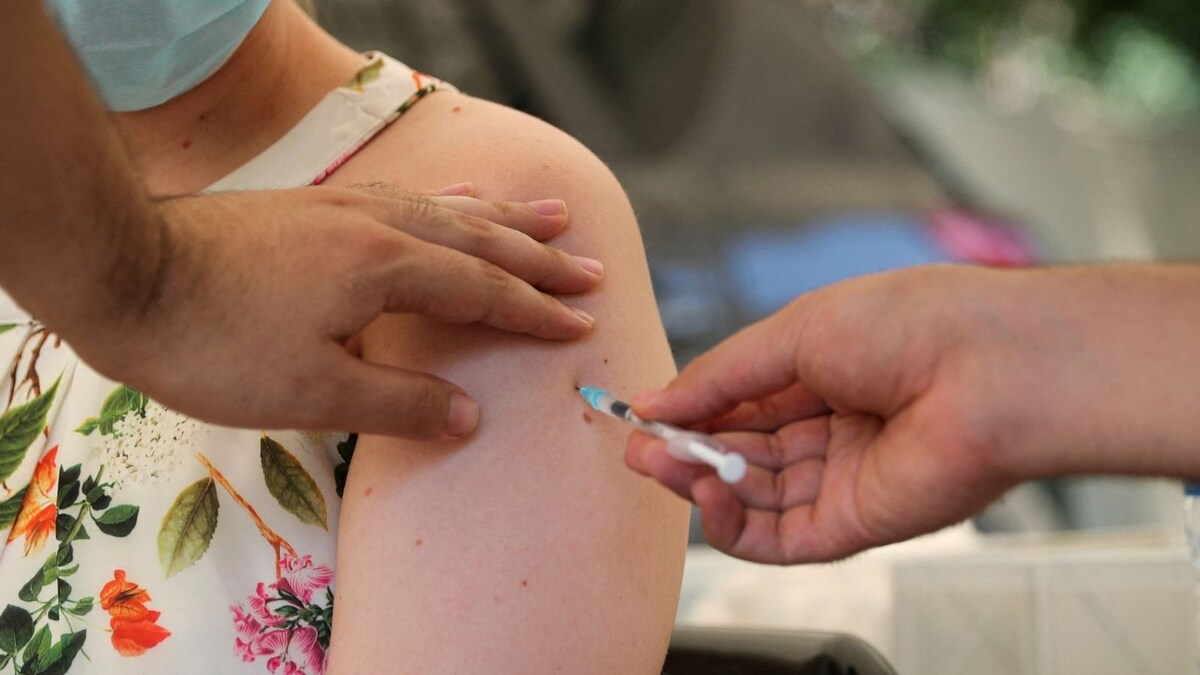
Govt Nod for Corbevax as Booster for Adults Vaccinated with Covishield, Covaxin Soon
News 18
Travelling to India from abroad? Govt mulls doing away with this rule
Live Mint
Bharat Biotech expects regulator’s nod for intranasal COVID-19 vaccine this month
The Hindu)
India supplied 23.9 crore COVID-19 vaccine doses to 101 countries, UN entities: Centre
Firstpost
India reports nearly 22,000 fresh covid cases, 60 deaths in last 24 hours
Live MintDiscover Related
)



















)