FDA authorizes 1st COVID-19 shots for infants, preschoolers
Associated PressU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week. The FDA also authorized Moderna’s vaccines for school-aged children and teens; CDC’s review is next week. While young children generally don’t get as sick from COVID-19 as older kids and adults, their hospitalizations surged during the omicron wave and FDA’s advisers determined that benefits from vaccination outweighed the minimal risks. “Both of these vaccines have been authorized with science and safety at the forefront of our minds,’’ Dr. Peter Marks, FDA’s vaccine chief, said at a news briefing. “It’s a real tragedy, when you have something free with so few side effects that prevents deaths and hospitalization,’’ said FDA Commissioner Robert Califf.
History of this topic

Sudden deaths among youth not linked to Covid vaccines, JP Nadda tells Parliament
India Today
Trump’s first term brought world-changing vaccine. His second could bring retreat
LA TimesDon’t wait for a holiday surge. Now is a good time to get your flu and COVID-19 vaccines
Associated Press
U.S. School Vaccination Rates Fall As Exemptions Keep Rising
Huff Post
California’s COVID surge is finally over. But expect another spike in the coming months
LA Times
Getting the COVID vaccine during pregnancy protects newborns from hospitalization
NPR
Florida's child vaccination rate plummets: "Kids aren't getting the protection they need"
SalonNew COVID-19 vaccines targeting JN.1 variant under review by Therapeutic Goods Administration
ABC
As COVID wave wallops California, new vaccines arrive this week. Will it be a turning point?
LA Times
The new COVID shot is now available. Here's what you need to know
NPR
Column: Opposing vaccine mandates, Trump exposes kids to disease
LA Times
Global childhood vax coverage stalled in 2023, 2.7 million kids unprotected, says UN
New Indian ExpressAs COVID-19 ticks up in some places, US health officials recommend a fall vaccination campaign
Associated Press
As COVID-19 ticks up in some places, US advisers recommend a fall vaccination campaign
The IndependentFDA advisers urge targeting JN.1 strain in recipe for fall’s COVID vaccines
Associated PressDo kids still need the COVID-19 vaccine in Australia?
ABC
'Defences tend to fade over time': US health agency recommends older to get another COVID shot this spring
India TV NewsOlder US adults should get another COVID-19 shot, health officials recommend
Associated Press
Older U.S. adults should get another COVID-19 shot, CDC recommends
LA TimesNewest COVID shots are 54% effective in preventing symptoms, CDC finds
Associated Press
JN.1 Covid variant: Health experts advise elderly to mask up
Hindustan Times
New COVID-19 vaccines available in Australia: Millions urged to get a booster as cases rise
Daily Mail
Learning from Covid-19 vaccination experience
Hindustan Times
Covid-19 vaccination rate is ‘lower than we’d like to see,’ CDC says
CNN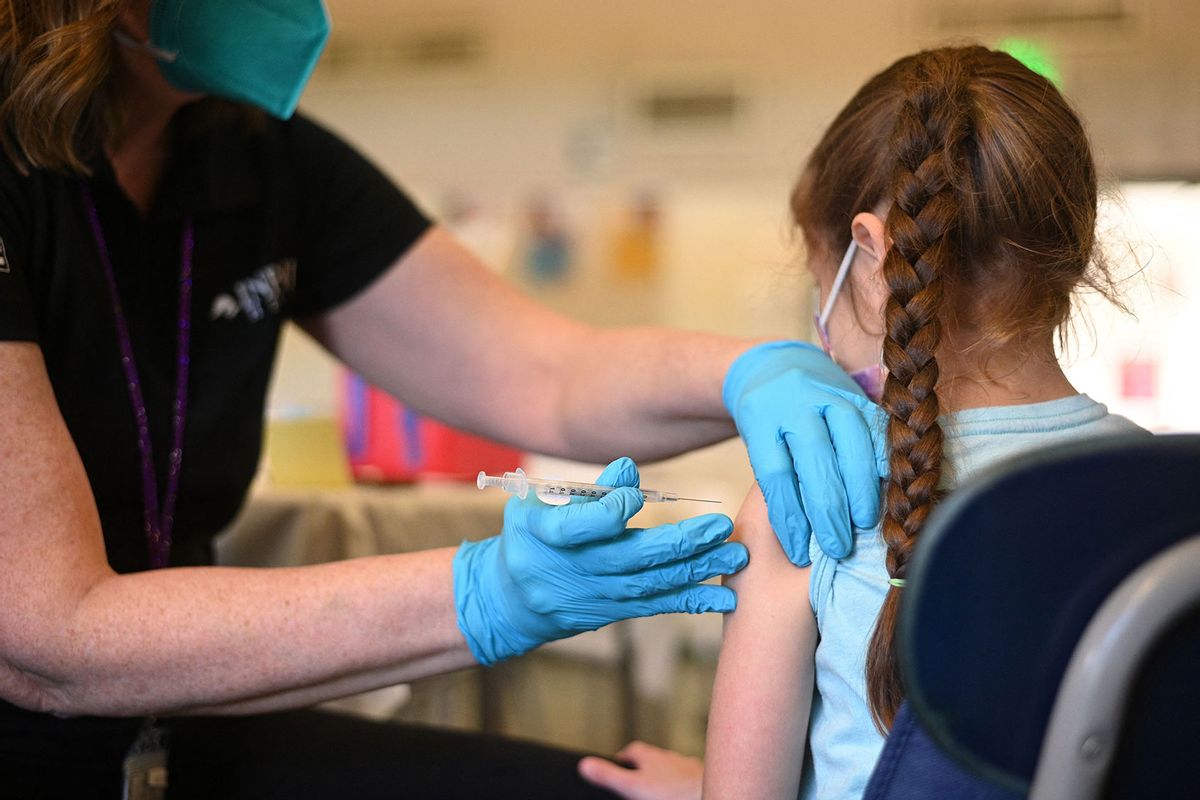
Few kids are getting the latest COVID shots. Is it misinformation or a lack of access?
Salon2% of kids and 7% of adults have gotten the new COVID shots, US data show
Associated Press
L.A. COVID-19 cases falling again after summer uptick. Officials now brace for winter
LA Times
CDC recommends updated Covid-19 vaccine for all, here's how you can get it
Hindustan Times
More COVID shots are coming. Will a weary public be more interested this year?
LA Times
COVID-19: US CDC recommends updated boosters for Americans, President Joe Biden calls it important milestone
India TV NewsAmericans can now get an updated COVID-19 vaccine
Associated Press
New COVID-19 vaccinations are coming, CDC says. The shots will likely be available this week
LA Times
Updated COVID vaccines will be available later this week, recommended for everyone six months and up
Salon
US FDA authorizes Pfizer-BioNTech, Moderna's updated Covid shots
India TodayU.S. approves updated COVID vaccines to rev up protection this fall
The HinduUS approves updated COVID vaccines to rev up protection this fall
Associated Press
Should you get the new COVID booster? The CDC is about to decide
LA Times
FDA approves updated COVID vaccines to rev up protection this fall
LA TimesUpdated COVID shots are coming. They’re part of a trio of vaccines to block fall viruses
Associated Press
FDA vaccine adviser Dr Paul Offit says healthy young people don't need another Covid booster - despite new BA.2.86 variant pushing virus rates up
Daily MailGov. DeSantis and Florida surgeon general warn against new COVID-19 restrictions and vaccine
Associated Press
New Covid booster shots to be approved in just TWO DAYS, sources suggest - but only 17% of Americans got the last one
Daily Mail
Australian Technical Advisory Group recommends COVID-19 booster for those over 75
Daily Mail
Biden says new Covid vaccines are 'likely' to be recommended to EVERY American this fall - including children - amid upswing in infections
Daily MailFDA approves RSV vaccine for moms-to-be to guard their newborns
Associated Press
With coronavirus uptick, should I get a COVID shot? When are new vaccines available?
LA TimesNew drug to protect babies and toddlers from RSV gets FDA approval ahead of cold season
Associated Press
Health council urges Covid booster for over 60s, care workers
Dutch News
CDC signs off on additional Covid-19 booster doses for certain people
CNN
Why millions of kids aren't getting their routine vaccinations
NPRDiscover Related

















)












)