FDA issues updated guidance on adapting Covid-19 vaccines, tests and therapeutics for coronavirus variants
CNNCNN — The US Food and Drug Administration has issued updated guidance for companies that plan to adapt their Covid-19 medical products – including vaccines, tests and therapeutics – to address the emergence of coronavirus variants. The agency said in an announcement on Monday that it expects that “manufacturing information will remain generally the same” for authorized Covid-19 vaccines that may be updated to target variants. “For clinical data, the guidance recommends that a determination of effectiveness be supported by data from clinical immunogenicity studies, which would compare a recipient’s immune response to virus variants induced by the modified vaccine against the immune response to the authorized vaccine,” the agency noted in its announcement. According to the FDA guidance, studies to assess the effectiveness of a Covid-19 vaccine’s primary shot and booster dose should compare the immune response induced by the modified version of the vaccine to the original vaccine, and researchers should conduct a “booster study” in which the modified vaccine is administered to those who previously received the original vaccine. FDA officials said during Monday’s call that some of the updated guidance for Covid-19 vaccines is modeled after what is already done for the development of seasonal influenza vaccines.
History of this topic

Coronavirus FAQ: I didn't get the latest COVID vaccine. Should I? And if so ... when?
NPR
Wondering If It's Too Late To Get A COVID Vaccine Before Thanksgiving? Read This.
Huff PostNew COVID-19 vaccines targeting JN.1 variant under review by Therapeutic Goods Administration
ABC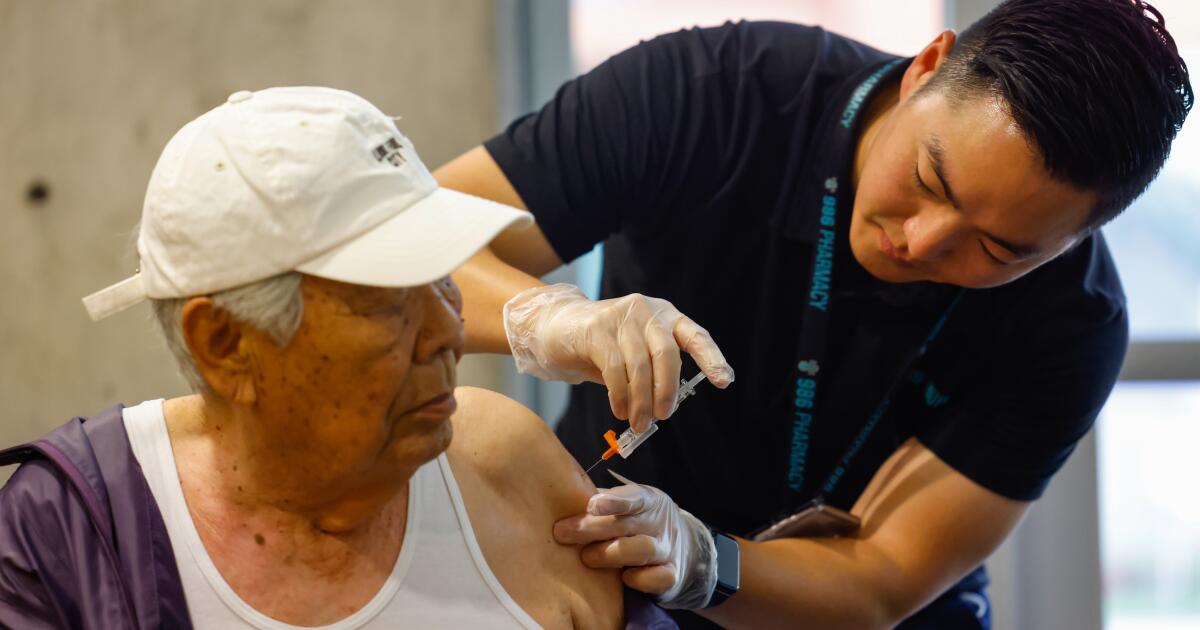
As COVID wave wallops California, new vaccines arrive this week. Will it be a turning point?
LA Times
An update on fall respiratory viruses
NPR
FDA approves updated COVID-19 vaccines; shots should be available in days
LA Times
The new COVID shot is now available. Here's what you need to know
NPR
COVID-19 variant KP.3.1.1 predominant in US as infections continue ticking up
China Daily
‘Playing COVID roulette’: Some infected by FLiRT variants report their most unpleasant symptoms yet
LA TimesAs COVID-19 ticks up in some places, US health officials recommend a fall vaccination campaign
Associated Press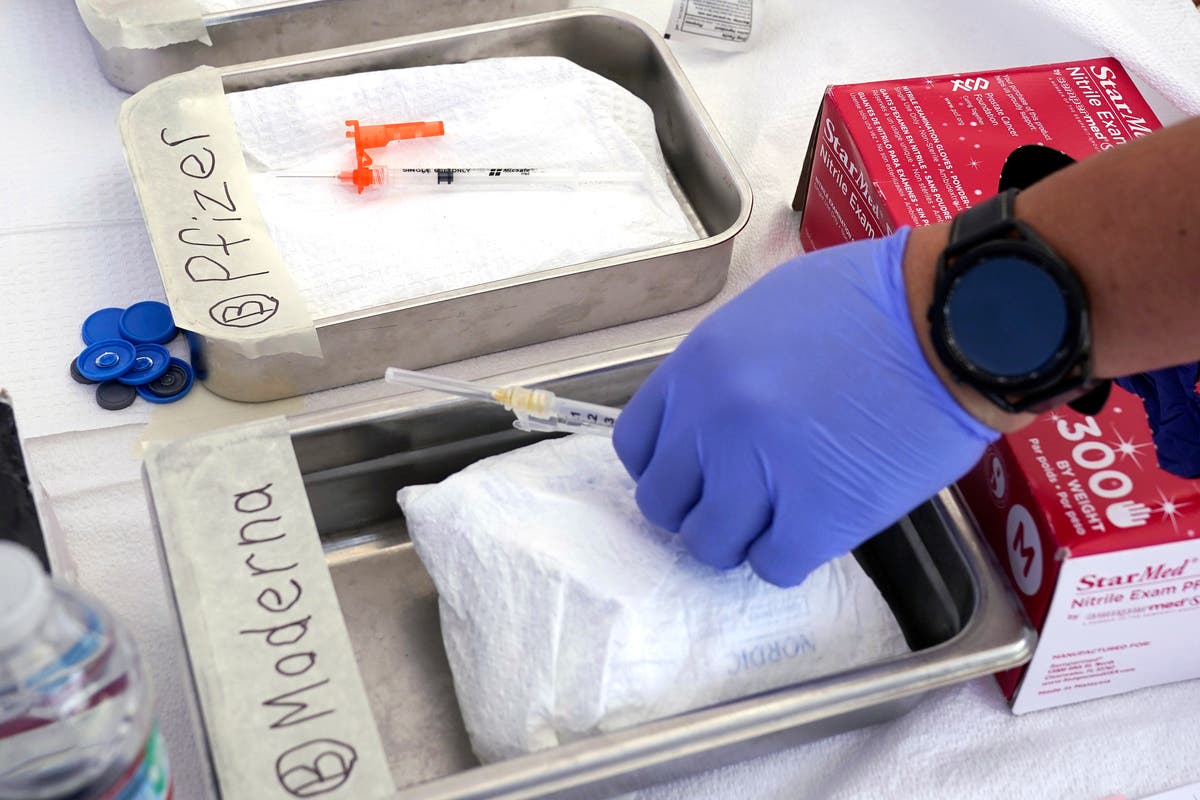
As COVID-19 ticks up in some places, US advisers recommend a fall vaccination campaign
The IndependentFDA advisers urge targeting JN.1 strain in recipe for fall’s COVID vaccines
Associated Press
There’s a new highly transmissible COVID-19 variant. Could FLiRT lead to a summer uptick?
LA Times
Does frequently updating COVID-19 vaccines have any benefits? | Explained
The Hindu
What you need to know as fall vaccinations against COVID, flu and RSV get underway
LA Times
Where Southern Californians can find the new COVID-19 vaccine
LA Times
When should I get vaccinated for COVID-19? Flu? RSV?
LA Times
COVID-19: US CDC recommends updated boosters for Americans, President Joe Biden calls it important milestone
India TV News
What To Know About New COVID Boosters And Who Is Eligible
Huff PostAmericans can now get an updated COVID-19 vaccine
Associated Press
New COVID-19 vaccinations are coming, CDC says. The shots will likely be available this week
LA Times
Updated COVID vaccines will be available later this week, recommended for everyone six months and up
Salon
US FDA authorizes Pfizer-BioNTech, Moderna's updated Covid shots
India TodayU.S. approves updated COVID vaccines to rev up protection this fall
The HinduUS approves updated COVID vaccines to rev up protection this fall
Associated Press
FDA approves updated COVID vaccines to rev up protection this fall
LA TimesUpdated COVID shots are coming. They’re part of a trio of vaccines to block fall viruses
Associated Press
FDA vaccine adviser Dr Paul Offit says healthy young people don't need another Covid booster - despite new BA.2.86 variant pushing virus rates up
Daily MailGov. DeSantis and Florida surgeon general warn against new COVID-19 restrictions and vaccine
Associated Press
Moderna says updated COVID vaccine is effective against ‘highly-mutated’ BA.2.86 variant
Hindustan Times
New COVID vaccine, due as early as next week, appears to work well against Pirola subvariant
LA Times
Biden says new Covid vaccines are 'likely' to be recommended to EVERY American this fall - including children - amid upswing in infections
Daily Mail
With coronavirus uptick, should I get a COVID shot? When are new vaccines available?
LA TimesNext round of COVID-19 shots in fall will target latest omicron strain
Associated PressFDA advisers endorse updating COVID vaccines to target latest omicron strain
Associated Press
An FDA panel meets about which COVID strains the U.S. will target with vaccines
NPR
FDA advisers back updated COVID shots for fall vaccinations
NPR
COVID vaccine should be updated to target XBB strain, FDA committee says
LA Times
CDC signs off on additional Covid-19 booster doses for certain people
CNN
No need for booster vaccine against coronavirus this spring, experts decide
Dutch News
No need for booster vaccine against coronavirus this spring, experts decide
Dutch News
FDA advisers set to consider one-and-done annual covid booster plan
Live Mint
FDA’s advisors back plan to simplify COVID-19 vaccinations
LA Times
FDA proposes once-a-year COVID-19 shots for most Americans
LA Times
FDA wants to simplify the use and updating of Covid-19 vaccines
CNN
FDA vaccine advisers ‘disappointed’ and ‘angry’ that early data about new Covid-19 booster shot wasn’t presented for review last year
CNN
NOT REAL NEWS: A look at what didn't happen this week
The Independent
As COVID-19 spikes in California, booster shots could make all the difference
LA Times
US renews push for COVID boosters as data show they protect
Associated Press
Panel votes to add COVID shots to recommended vaccinations
Associated PressDiscover Related










)