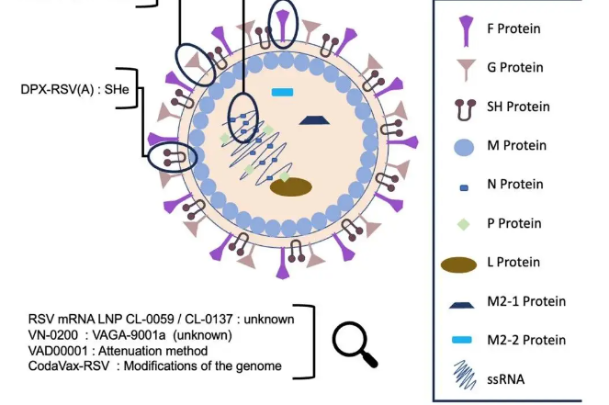
Serum-Oxford Covid-19 vaccine gets DCGI nod for phase 2, 3 clinical trials in India
Live MintNew Delhi: India's top drug regulator-- Drugs Controller General of India has granted permission to Serum Institute of India to conduct phase 2 and 3 human clinical trials in India on the potential COVID19 vaccine, a senior government official said. And after considering the data generated on the vaccine in phase-1, 2 of the Oxford University trial, the committee recommended granting permission to conduct phase 2, 3 clinical trials of COVISHIELD healthy adult subjects at risk in the country," the official said. Last week ANI had reported that the domestic pharma giant had made an application to DCGI for grant of permission to conduct phase 2, 3 clinical trials of coronavirus vaccine in India. Currently, phase 2, 3 clinical trials of the Oxford sponsor vaccine is ongoing in United Kingdom, phase 3 clinical trial in Brazil and phase 1, 2 clinical trials in South Africa.
History of this topic

Recent breakthrough in COVID-19 vaccination: Indian Immunologicals Ltd unveils first needle-free booster vaccine
Hindustan Times
Serum Institute 'hopeful' of producing Mpox vaccine, says CEO Adar Poonawalla
India TV News
Serum Institute of India's new 'high efficacy' malaria vaccine rolls out in Africa
India Today
AstraZeneca's Covishield Showed Lowest Incidence of Side Effects in India Versus Globally, Says Top Govt Official
News 18
After AstraZeneca covid-19 vax recall worldwide, Serum says it stopped manufacturing jabs in Dec 2021
Live Mint
Stopped manufacturing Covishield since December 2021, says Serum Institute
Hindustan Times
Only 7 Out 10 Lakh May Face Clotting Risk Due to Covishield: Former ICMR Scientist
News 18
AstraZeneca reaffirms Covid vaccine safety amid rare side effect concerns
India TV News
With State prod, India can be world leader in vaccines
Hindustan Times
Serum Institute of India joins global network to boost production of affordable outbreak vaccines
The Hindu
COVID-19 Update: India records 260 new cases, total active at 1,828
Live MintOxford-Serum institute malaria vaccine recommended for use by WHO
The Hindu
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
India urges G-20 countries to join vaccine research collaborative in wake of COVID vaccine inequity
The Hindu
Explained | What are the problems with India’s clinical trials registry?
The Hindu
Serum Institute could resume Covishield production as Covid cases rise
Live Mint
Serum institute seeks to add Covovax on CoWIN portal as booster dose for adults
Hindustan Times)
‘The Vial’ trailer out: Manoj Bajpayee narrates History TV18's documentary on India’s Covid-19 vaccine journey
Firstpost
Covid vaccine mission is a lesson for the world
Hindustan Times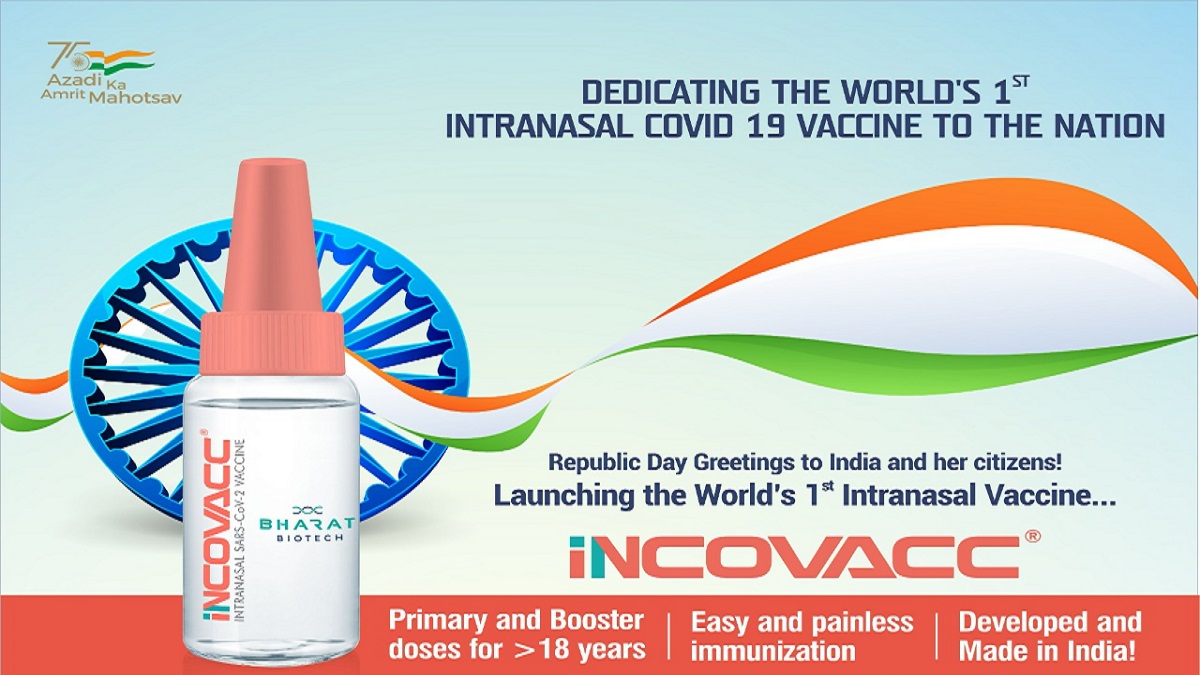
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News
India launches world’s 1st intranasal Covid vaccine: Check price, availability
Hindustan Times
Health minister Mandaviya launches Bharat Biotech's nasal Covid vaccine
Hindustan Times
COVID-19: Mansukh Mandaviya launches world's 1st Made-in-India nasal vaccine iNCOVACC
India TV News
Bharat Biotech’s nasal vaccine likely to be launched on Jan 26
Hindustan Times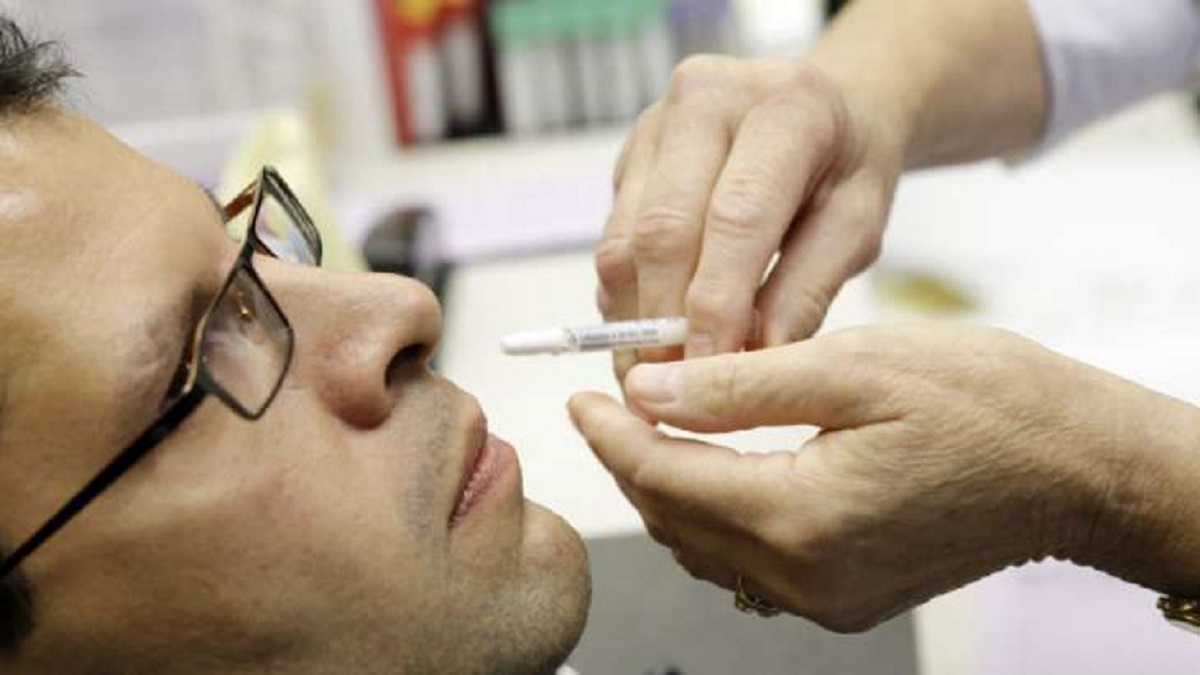
First Indian intranasal Covid vaccine by Bharat Biotech to be launched on Jan 26
India TV News
DCGI Approves Market Authorisation for SII's Covid Vaccine Covovax as Heterologous Booster Dose
News 18
No second Covid-19 booster dose required: Report
Live Mint
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The Hindu
Price of Bharat Biotech’s nasal Covid vaccine revealed. Details here
Live Mint)
COVID-19: Centre approves Bharat Biotech's intranasal vaccine, to be available on CoWIN
Firstpost
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News
SII Seeks Drug Regulator's Nod for Market Authorisation of Covovax Vaccine as Covid-19 Booster Dose
News 18
Three reasons India will not see a Covid-wave like China’s
Hindustan Times
A concerning sequence: The Hindu Editorial on a new coronavirus strain and a spurt in cases in China
The HinduIndia need not panic given excellent COVID-19 vaccination coverage: Adar Poonawalla
The Hindu
'Rising Covid cases in China concerning,' says Serum Institute's Adar Poonawalla
Hindustan Times
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
Oxford, Serum initiative: Anti-malaria vaccine’s Phase 3 trials conclude
Hindustan Times
World’s first intra-nasal vaccine for COVID gets CDSCO nod for restricted use
Live Mint
What Makes India's Covid Vaccination Programme A Global Best Practice In Public Health
News 18
Health Ministry not to procure fresh COVID-19 vaccines; surrenders ₹4,237 crore from vaccination budget
The Hindu
AstraZeneca Nasal Spray Vaccine for Covid-19 Suffers Setback in Early Trial
News 18VINCOV-19, country’s first anti-dote for COVID, ready for phase 3 trials, market authorisation
The Hindu
Covid-19: India logs nearly 5,000 new infections, active cases drop below 45k
Live Mint
Will Two ‘Made-in-India’ Vaccines Be the New Covid Boosters? News18 Decodes Differences
News 18
Bharat Biotech’s intra-nasal COVID vaccine gets emergency use approval
The Hindu
India's First Nasal Vaccine for Covid-19, Made by Bharat Biotech, Cleared for Emergency Use
News 18
Covid-19: India's first intranasal vaccine by Bharat Biotech gets DCGI approval
India TV News
Monkeypox vaccines: India puts emergency buying on hold for now - here’s why
Live Mint)
Cervical cancer vaccine to be launched in few months
FirstpostDiscover Related
)





















)