Jubilant, Gilead ink pact for Remdesivir
The HinduJubilant Life Sciences, through a subsidiary, has entered into a non-exclusive licensing agreement with Gilead Sciences Inc. to register, manufacture and sell Gilead’s Remdesivir, a potential therapy for COVID-19 in 127 countries, including India. Jubilant Life Sciences chairman Shyam S.Bhartia and co-chairman and MD Hari S. Bhartia said Remdesivir, based on initial data, showed promise as a potential therapy for COVID-19. We also plan to produce the drug’s active pharmaceutical ingredient in-house helping its cost effectiveness and consistent availability.” An investigational anti-viral therapy developed by Gilead, Remdesivir received Emergency Use Authorisation by the US Food and Drug Administration to treat COVID-19. The EUA was based on available data from two global clinical trials – U.S. National Institute for Allergy and Infectious Diseases’ placebo-controlled Phase 3 study in patients with moderate to severe symptoms of COVID-19 and Gilead’s global Phase 3 study evaluating Remdesivir in patients with severe disease.
History of this topic

Gilead Put Lives before Profits! Says AHF INDIA
Deccan Chronicle
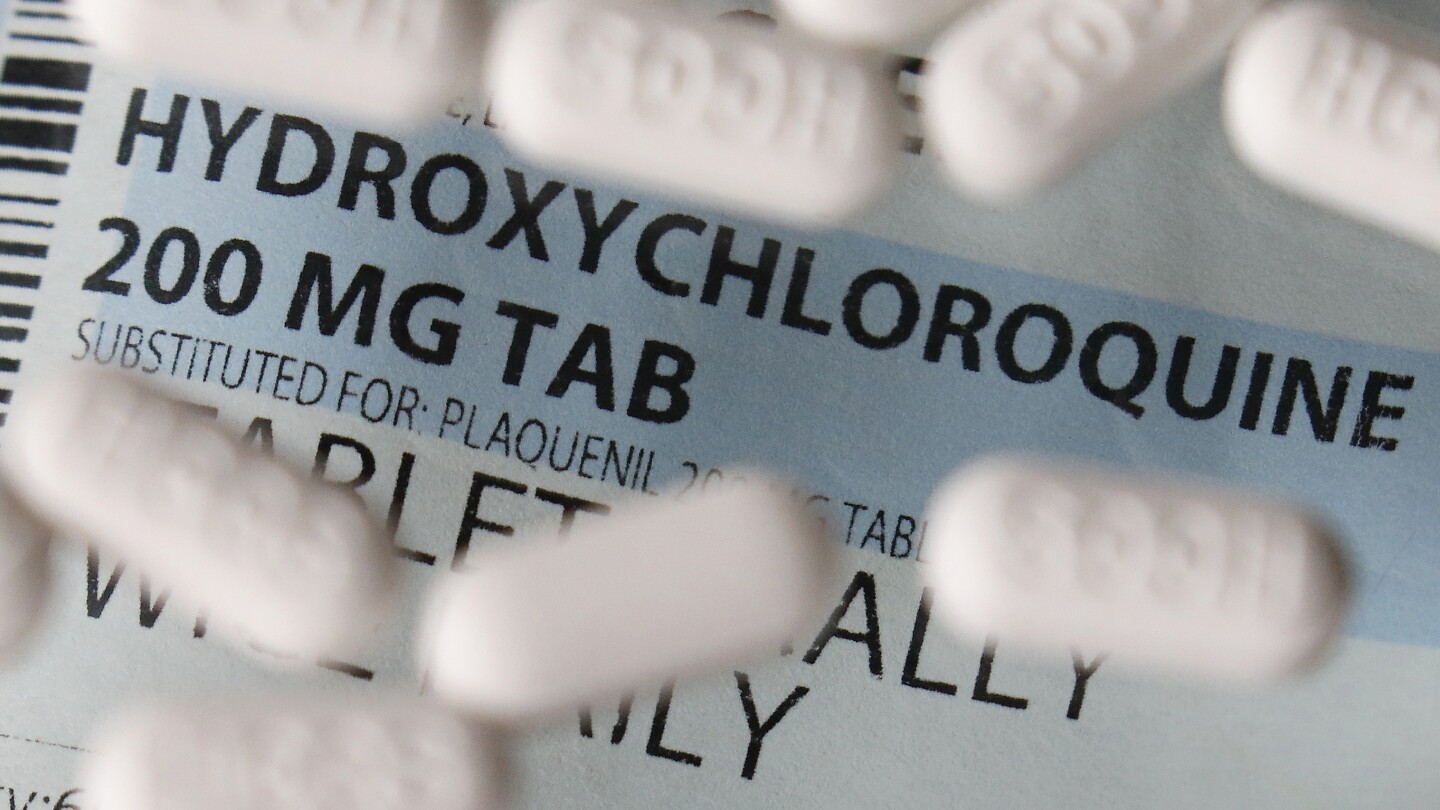
FDA revokes authorization for key anti-COVID drug, a blow for vulnerable Americans
LA Times
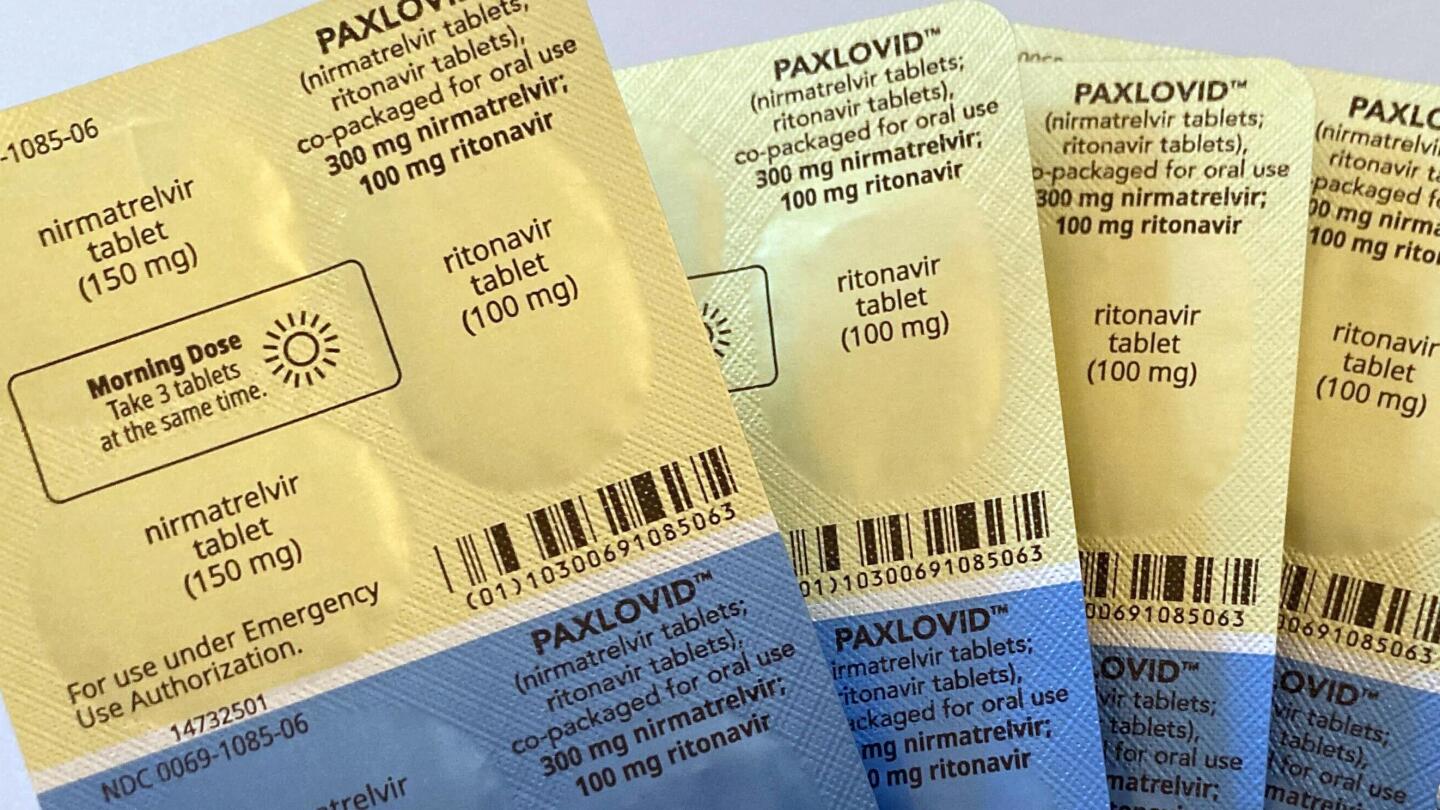
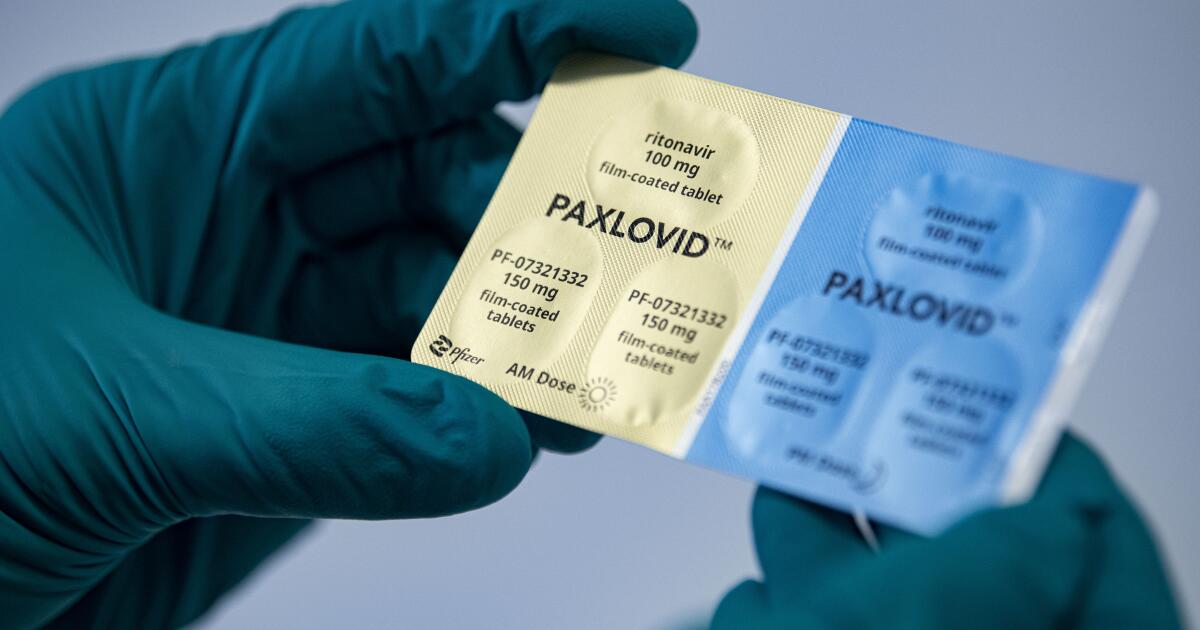
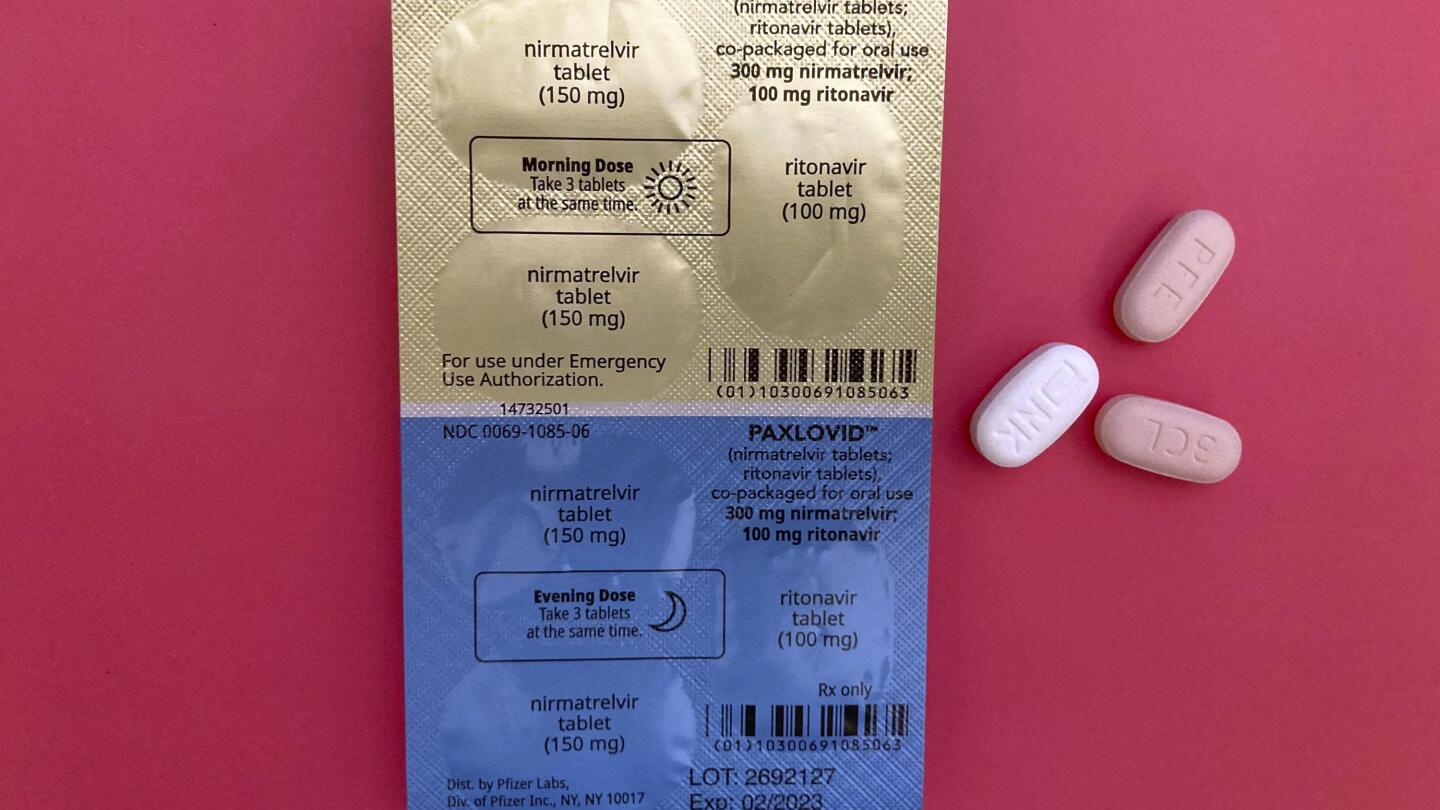
WHO recommends Pfizer's pill Paxlovid for mild Covid cases
India Today
Health Matters: Dear Drug Regulator, Silence is Not Always Golden, Esp on WHO Pausing Covaxin Supply
News 18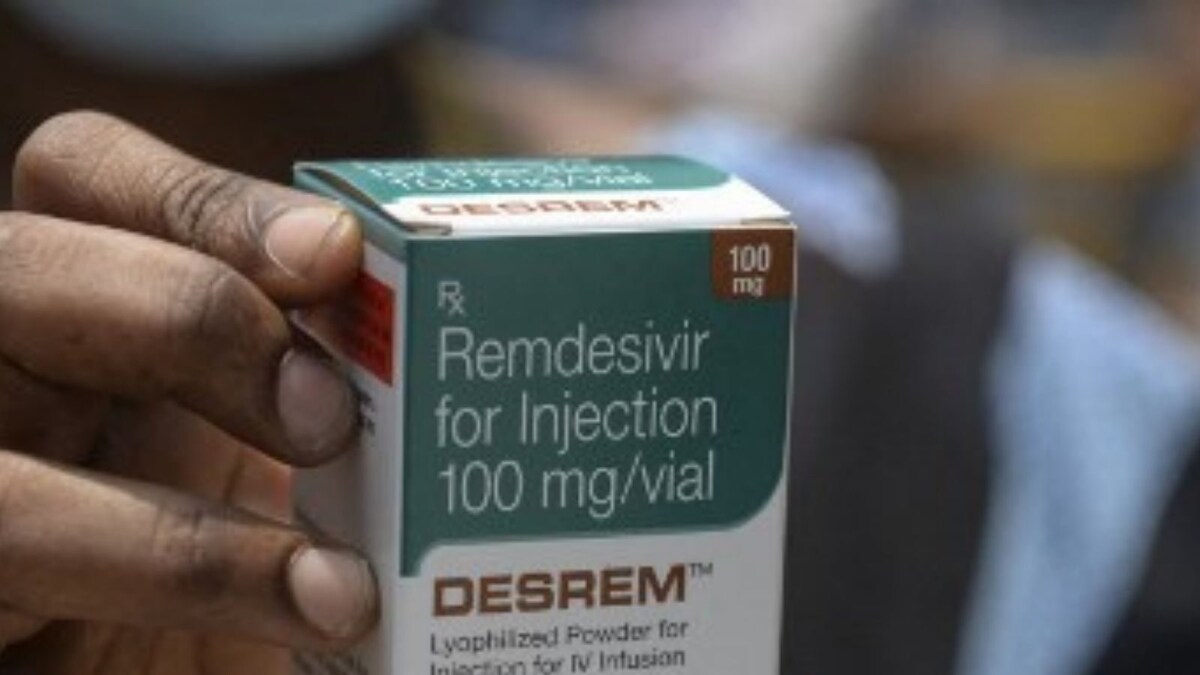
Why Remdesivir’s Manufacturing Licence was Not Given to Pharma PSUs When People Were Battling Second Wave: Parl Panel
News 18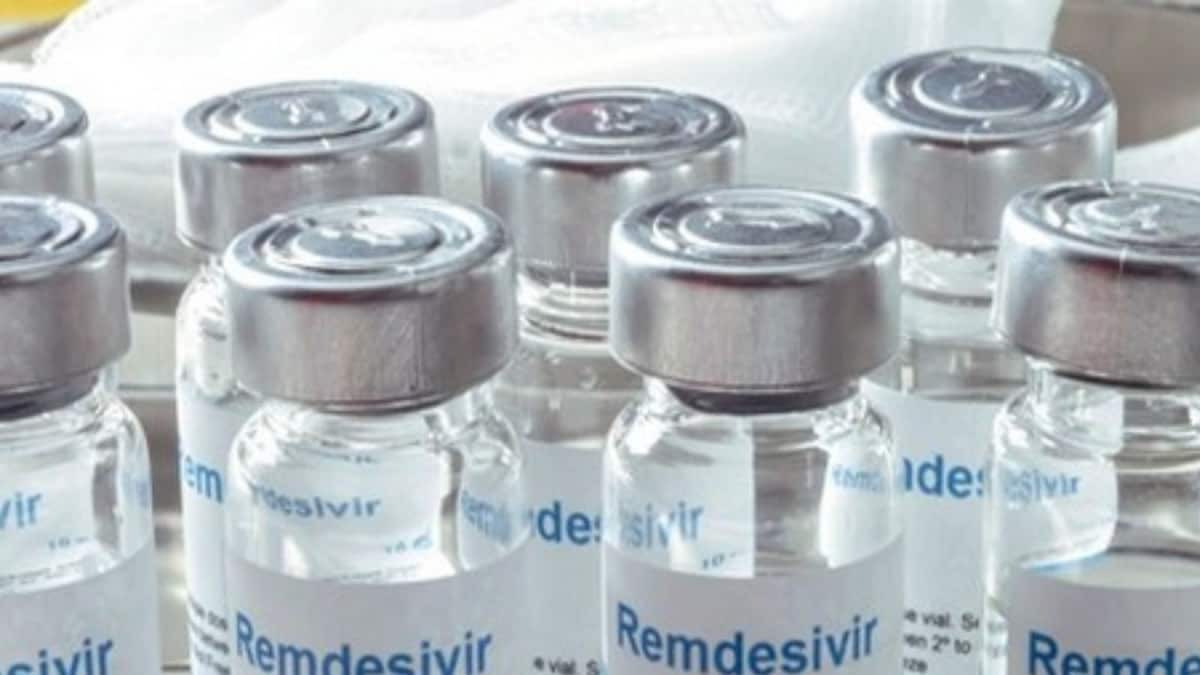
Health Matters: Don't Feel Guilty About Not Finding Remdesivir for Your Loved One. It Hardly Works
News 18)
New COVID-19 treatment rules: India cuts down use of Remdesivir, Tocilizumab, multivitamins, steroids
Firstpost
India Cuts Use of Remdesivir, Tocilizumab, Multivitamins, Steroids in Covid-19 Treatment
News 18
A shot in the arm: The Hindu Editorial on new modes of COVID-19 treatment
The Hindu
Pfizer Covid-19 antiviral pill: Biden says he’s ‘encouraged by the promising data’
CNN
Merck’s COVID-19 pill narrowly wins FDA panel’s support
LA Times
EU regulator recommends Merck’s COVID-19 pill for emergency use
Al Jazeera
Pfizer says COVID-19 pill cut hospital, death risk by 90%
Associated Press
UK approves Merck’s antiviral COVID-19 pill in world first move
Al Jazeera
Molnupiravir: UK authorizes Merck/Ridgeback Biotherapeutics’ antiviral to treat mild-to-moderate Covid-19
CNN
Merck seeks first US authorization for Covid-19 tablet
Live Mint
Merck seeks US authorisation for pill to treat COVID
Al JazeeraExplained | Molnupiravir, Merck’s new drug to treat COVID-19
The Hindu
Merck Covid pill: What would an antiviral pill mean for the fight against Covid-19?
CNN
Biological E's ‘Corbevax’ gets DCGI approval for two clinical trials
Hindustan Times)
Anti-Diabetic Drugs Have Potential to Treat Covid-19, Finds Hyderabad University Study
News 18
US to spend $3.2B on treatments for COVID-19, other viruses
Associated Press
Biden administration will spend $3.2 billion on development of antiviral pills for treating COVID-19
Daily Mail)
DGHS issues COVID-19 treatment guidelines: Here is what document recommends on Remdesivir, Tocilizumab
Firstpost)
Govt Issues Advisory for Rational Use of Remdesivir in Covid-19 Treatment
News 18)
No evidence of Remdesivir's effectiveness, might be dropped as COVID-19 treatment: Dr DS Rana
Firstpost
Remdesivir Sale To Be Shifted To Chennai Nehru Indoor Stadium
The Quint
Coronavirus Fact Check: Is Remdesivir life saving drug in COVID19, can it be given to patients at home?
India TV News
When to use Remdesivir, Ivermectin? India's Covid treatment protocol and what experts say
India Today
Eli Lilly signs licensing pact with Cipla, Sun, Lupin for Covid-19 drug
Business Standard)
What Widespread Recommendation of Remdesivir for COVID-19 and Its Shortage Teach Us
News 18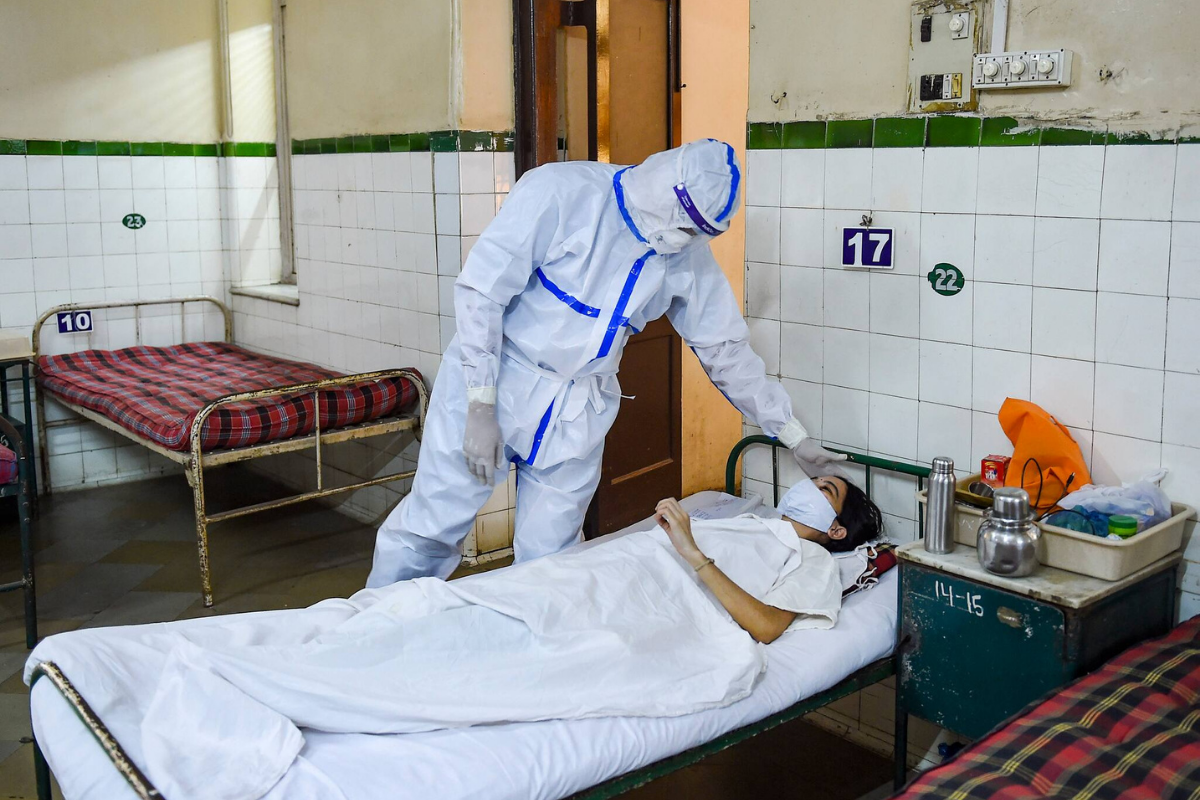)
COVID-19 Treatment: Cipla to manufacture and sell Eli Lilly's baricitinib in India
News 18)
Covid-19: Remdesivir Allocation Made Up to May 16 to Ensure Availability, Says Union Minister
News 18Doctors divided over use but scramble for Remdesivir continues
The Hindu
COVID: Remdesivir production expected to start in Wardha from Wednesday, says Nitin Gadkari
India TV News
American biopharmaceutical company Gilead announces steps to expand Remdesivir availability in India
India TV News
Russian firm awaits government approval to ship remdesivir to India
India Today)
'Not a Life-Saving Drug': ICMR Suggests Safe Use of Remdesivir in Covid-19
News 18)
Remdesivir Not Magic Bullet, Does Not Reduce Covid-19 Mortality: AIIMS Chief Randeep Guleria
News 18)
Amid Covid Spurt, Medicos Advice People To Use Remdesivir Judiciously and Only in a Hospital Setting
News 18
Jharkhand seeks Centre's nod to import Remdesivir vials from Bangladesh
Hindustan Times
Why Indian doctors prescribe Remdesivir for Covid-19 despite negative global medical view
India Today
When Do You Really Need Remdesivir? Does It Work?
The Quint
Covid-19: Pune asks Mumbai for 20,000 vials of Remdesivir amid vaccine shortage
India Today)
Amid Shortage, Remdesivir May Get Cheaper as Govt Says Manufacturers Offered to Cut Rate by Sunday
News 18
Remdesivir: What is it and why is it being black marketed? All you need to know
Op India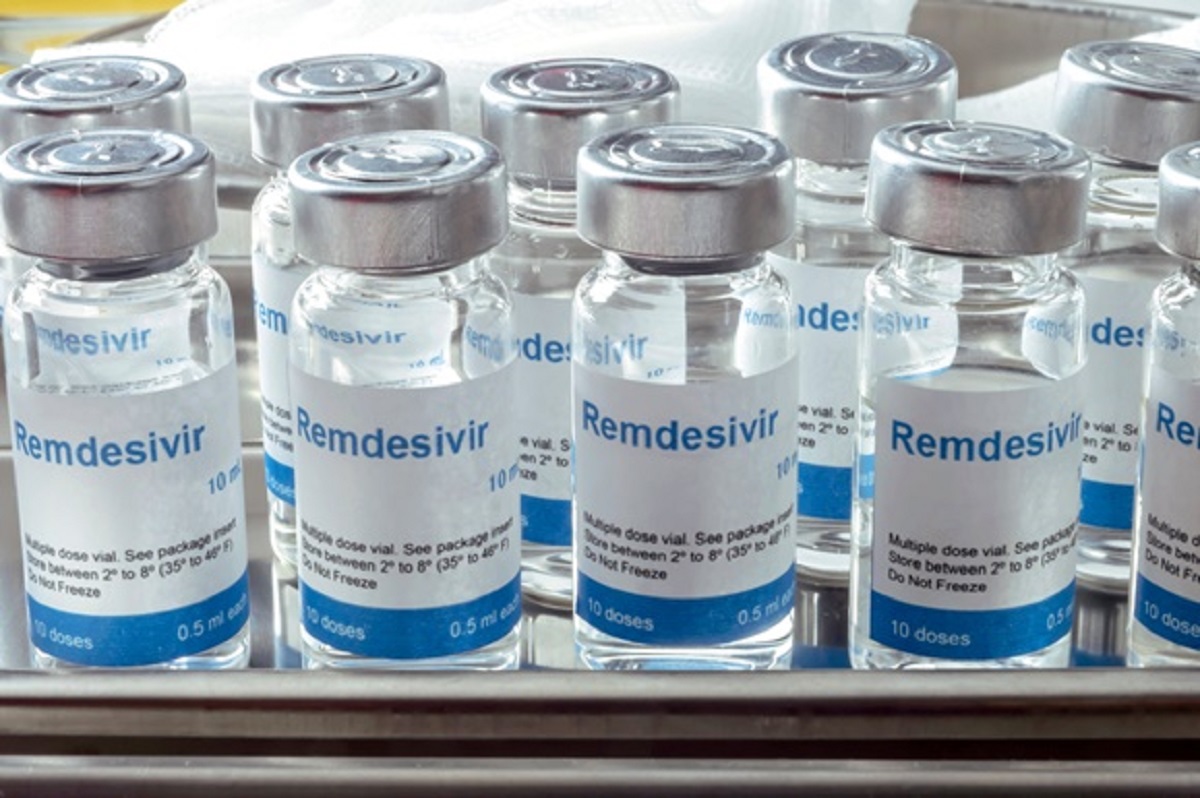
Yogi Adityanath directs UP health department to urgently acquire Remdesivir injections from Ahmedabad
India TV News
Remdesivir Explained: What is the repurposed Covid-19 drug, why is there a shortage, do you need it?
India Today
No evidence of Remdesivir's effectiveness in treating Covid patients: WHO
India TodayCoronavirus | India bans export of Remdesivir
The HinduDiscover Related






























)