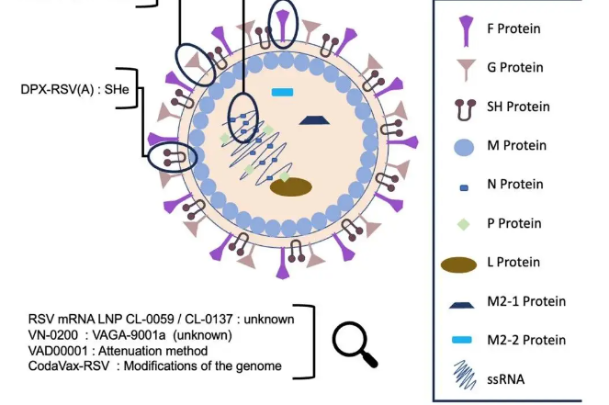
The second dose: The Hindu Editorial on COVID-19 vaccine clearance in India
The HinduWith the U.K. regulator granting an emergency-use authorisation for AstraZeneca’s COVID-19 vaccine on December 30, three vaccines have now been greenlighted for use even before a full approval is granted. While published results show only 62% efficacy when two full-strength doses are administered four weeks apart, the U.K regulator has relied on unpublished data showing efficacy increasing to 73%, 22 days after the first dose when the second jab was delayed. With the virus running amok, the U.K. has revised its policy to delay the second dose of the Pfizer vaccine too despite no trial data to support this; the second Pfizer jab is originally scheduled three weeks after the first. If the pandemic forced scientists, companies and regulators to quickly make available safe and efficacious COVID-19 vaccines, delaying the second dose to maximise the benefits is altogether a new strategy. Since delaying the second dose appears to be increasing the efficacy, and as the U.K. has approved a delayed second dosage, India should carry out large trials to test this.
History of this topic
India sees single-day rise of 702 COVID-19 cases
The Hindu
Over 220 crore COVID vaccine doses administered across India, but less than 23 crore booster doses: Health Ministry data
The Hindu
New COVID variant JN.1: Health Ministry says no need for booster dose
The Hindu
At least single dose vaccine prior to COVID-19 infection provided 60% protection against post-discharge mortality: ICMR study
The Hindu
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The HinduIndia sees over 7,800 COVID cases in a day reported on April 12, 2023
The Hindu
‘Study on covid vaccines’ efficacy soon’
Live Mint)
India sees a surge in COVID-19 cases: Does the country need a fourth jab?
Firstpost
After 113 days, India sees jump in Covid cases as 524 fresh infections surface in 24 hours
India TV News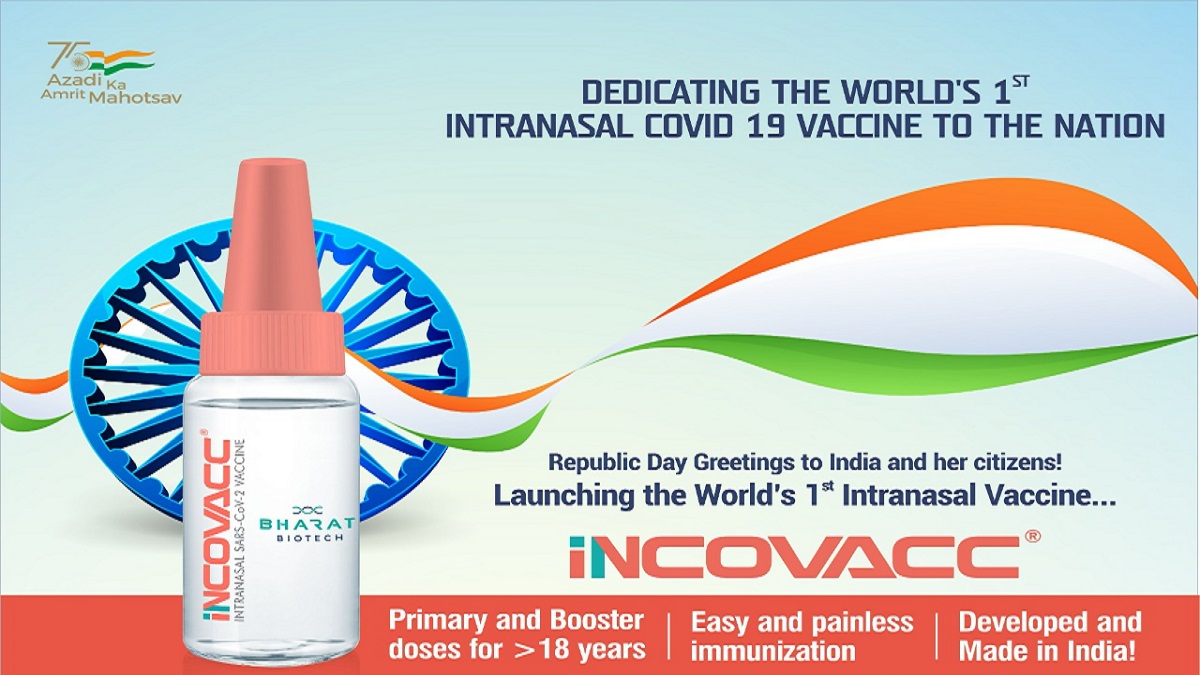
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News
Over 7,000 Doses of Bharat Biotech's Intranasal Jab Ready for Market Rollout as Apex Lab Clears Batch 1
News 18
Not Permissible To Take One Dose Of Covishield And Another Of Covaxin: Centre To Delhi High Court
Live Law
COVID-19: Mansukh Mandaviya launches world's 1st Made-in-India nasal vaccine iNCOVACC
India TV News
Time to Join this ‘Herd’: 39% Have Boosted Their Protection against Covid Virus, Finds Survey
News 18)
Second COVID-19 booster dose not required, says government sources
Firstpost
No second Covid-19 booster dose required: Report
Live Mint
Fourth Covid vaccine unwarranted right now but increased surveillance needed: experts
Deccan Chronicle
Bharat Biotech’s intranasal covid vaccine priced at ₹325 for govt hospitals
Live Mint
'Consider 2nd Booster': A Request Given to Mansukh Mandaviya During Covid-19 Meeting with Doctors
News 18
After Slow Start, Demand for Covid-19 Booster Shots Spikes as China's New Wave Triggers Panic in India
News 18
Your Best Shot at Fighting Covid: 28% Have Already Taken Booster Dose, Finds Survey
News 18
Indian Army issues advisory on Covid, asks defence personnel to take safety measures
India TV News
No Wastage of Covid Vaccines in Govt Buffer Stock Due to Expiry: Govt in Lok Sabha
News 18Explained | Can vaccine distribution be made fairer?
The Hindu
Health Ministry not to procure fresh COVID-19 vaccines; surrenders ₹4,237 crore from vaccination budget
The Hindu
India Reports 2,678 New Covid-19 Cases, 10 Deaths in a Day
News 18
AstraZeneca Nasal Spray Vaccine for Covid-19 Suffers Setback in Early Trial
News 18
Covid-19: India logs nearly 5,000 new infections, active cases drop below 45k
Live Mint
Will Two ‘Made-in-India’ Vaccines Be the New Covid Boosters? News18 Decodes Differences
News 18
COVID-19: Spain to start administering 4th dose of vaccine
India TV News)
India logs 5,379 new COVID-19 cases, daily positivity rate at 1.67%
Firstpost
Covid-19: India's first intranasal vaccine by Bharat Biotech gets DCGI approval
India TV News
An End to Foreign-return Vaccine Woes: India to Offer Remaining Covid-19 Shots Domestically
News 18
India logs 11,539 fresh Covid-19 cases, active cases below one-lakh mark
Live Mint
Tackling the new variants
Hindustan Times
Corbevax Likely to be Available As Covid Booster Dose at Vaccination Centres from Friday
News 18
Corbevax as Covid booster for adults from August 12. Check details
India Today
Corbevax approved as booster for those jabbed with Covishield, Covaxin: Report
Live Mint
In A First, Biological E's Corbevax Approved As Covid Booster for Covishield, Covaxin Recipients
News 18
Govt Nod for Corbevax as Booster for Adults Vaccinated with Covishield, Covaxin Soon
News 18
Travelling to India from abroad? Govt mulls doing away with this rule
Live Mint
Go For More Tests, Faster Vaccination: Worried by Uptick in Covid Graph, Centre's Advisory to 7 States
News 18
Covid19 vaccine: Centre reduces gap between 2nd and precaution dose to 6 months on NTAGI's advice
India TV News
Covid-19 update: India sees another rise with 19,893 cases in 24 hrs
Live Mint)
India records 19,673 new COVID-19 cases, daily positivity rate at 4.96%
Firstpost)
India supplied 23.9 crore COVID-19 vaccine doses to 101 countries, UN entities: Centre
Firstpost
India reports nearly 22,000 fresh covid cases, 60 deaths in last 24 hours
Live Mint)
India logs 20,528 new COVID-19 infections in 24 hours; active cases increased to 1,43,449
Firstpost
’Only 10% got Covid-19 booster dose’: CM Arvind Kejriwal’s appeal to Delhi
Live Mint
Milestone! India crosses 2 billion Covid-19 vaccination mark in 18 months
India TV NewsDiscover Related