Dr Reddy’s asked to resubmit application for both phase 2,3 trials of Russian COVID-19 vaccine Sputnik V
Live MintNew Delhi: An expert panel at the Central Drugs Standard Control Organisation has asked Dr Reddy's Laboratories to submit a revised protocol for conducting both phase 2 and phase 3 human clinical trials for the Russian vaccine against COVID-19, Sputnik V, in India, sources said Monday. The Hyderabad-based pharmaceutical company had applied to the Drugs Controller General of India late last week, seeking permission to conduct phase-3 human clinical trials of the Russian vaccine. The Subject Expert Committee on COVID-19 at the CDSCO, which held its meeting on Monday, deliberated on the application and asked the firm to submit a revised protocol stating it will have to conduct combined phase 2 and 3 clinical trials, sources told PTI. Currently two indigenously developed vaccine candidates, one by Bharat Biotech in collaboration with ICMR and another one by Zydus Cadila Ltd, are in the phase 2 of the human clinical trials.
History of this topic

1st Dose to Be Given as Booster to Those Vaccinated with Sputnik V, NTAGI Recommends
News 18
1st dose of Sputnik V to be given as booster to those vaccinated with Russian vaccine
India Today
Russia registers nasal version of Sputnik V, world's first nasal vaccine against COVID-19
India Today
Ukraine war: WHO delays review of Russia’s Sputnik V vaccine
Hindustan Times
Govt panel recommends permission for phase 3 trial of Sputnik Light vaccine as booster dose
India Today
Covid-19 Vaccine Sputnik V's India Production In A Fix After Sanctions on Russia's Jab Manufacturer
News 18
Omicron variant Updates: Religious places reopen in Bihar's Patna, Covid restrictions relaxed
India TV News)
DCGI grants emergency use permission to Sputnik Light COVID-19 vaccine
Firstpost
Single-dose Sputnik Light Covid vaccine gets approval for emergency use in India
India Today)
Government panel recommends emergency use authorisation for single-dose COVID-19 jab Sputnik Light
Firstpost
Govt panel recommends granting emergency use authorisation to single dose of Covid vaccine Sputnik Light
India Today
Trying to Bring Sputnik M Vaccine for Adolescents to India: Dr Reddy's
News 18
Sputnik V effective against Omicron, say developers
Hindustan Times
Expert panel may approve Sputnik Light vaccine for emergency use, discuss trials nasal vax
India Today
Sputnik V, Sputnik Light Booster Effective Against Omicron, Says RDIF
News 18
Russia says Sputnik-V vaccine effective against Omicron variant of coronavirus
India Today)
Russian president Vladimir Putin tests experimental COVID-19 nasal vaccine
Firstpost
What Went Wrong with Full Rollout of Russia’s Sputnik V Vaccine in India
News 18
Sputnik V Developer Calls for Mandatory Covid-19 Vaccines in Russia as Inoculation Remains Low
News 18
Dr Reddy's Expected to Submit Sputnik V Light Trial Data to DCGI in November
News 18
Serum Institute seeks DCGI nod for Covid vaccine Covovax
India Today
South African Regulator Rejects Russia's Sputnik V Covid Vaccine Citing Safety Concerns
News 18
Russia struggles to meet global orders for Sputnik V vaccine
Associated Press
Russia, WHO differ on when approval will come for Sputnik V
Associated Press
One-shot Sputnik Light Vaccine Shows 70% Efficacy Against Covid Delta Variant: RDIF
News 18
Centre permits export of 40 lakh doses of Covid-19 vaccine Sputnik Light manufactured in India to Russia
India Today
India Allows Export of Russia’s Sputnik Light Covid Jab Produced by Hyderabad’s Hetero Biopharma
News 18
Govt allows export of Russia’s Sputnik Light Covid-19 vaccine made in India
Live Mint
Russians flock to Serbia for Western-made COVID-19 vaccines
Associated Press)
Data on Russia's Sputnik V vaccine is still under review for emergency use approval: WHO
Firstpost
WHO still reviewing Sputnik V vaccine, as Russia presses bid
Associated Press
WHO Still Reviewing Sputnik V Vaccine For Emergency Use As Russia Presses Bid
News 18)
Russia seeks Indian government's nod for export of Sputnik Light COVID-19 vaccine
Firstpost)
Sputnik Light, J&J take another step towards joining India's COVID vaccine arsenal
Firstpost
Dr Reddy’s Labs gets approval for phase 3 trials of Sputnik Light in India
Hindustan Times
India to Begin Testing Russia’s Single-Shot Sputnik Light Shortly, 1st Tranche Sent for Quality Testing
News 18
Covid-19 Vaccine: Dr Reddy's Starts Supply of Sputnik V First Doses
News 18
Sputnik V, AstraZeneca Vaccine Cocktail Shows High 'Immunity Profile': Study
News 18
Sputnik V and AstraZeneca vaccine cocktail shows positive results in early trials
India Today
Sputnik V hits production roadblock for second dose of Covid vaccine | Exclusive
India Today
AstraZeneca, Sputnik V \'mix & match\' vaccine joint trials show no adverse events
Deccan Chronicle
AstraZeneca-Sputnik mixing: No breakthrough infection, no serious side effects | Study
India Today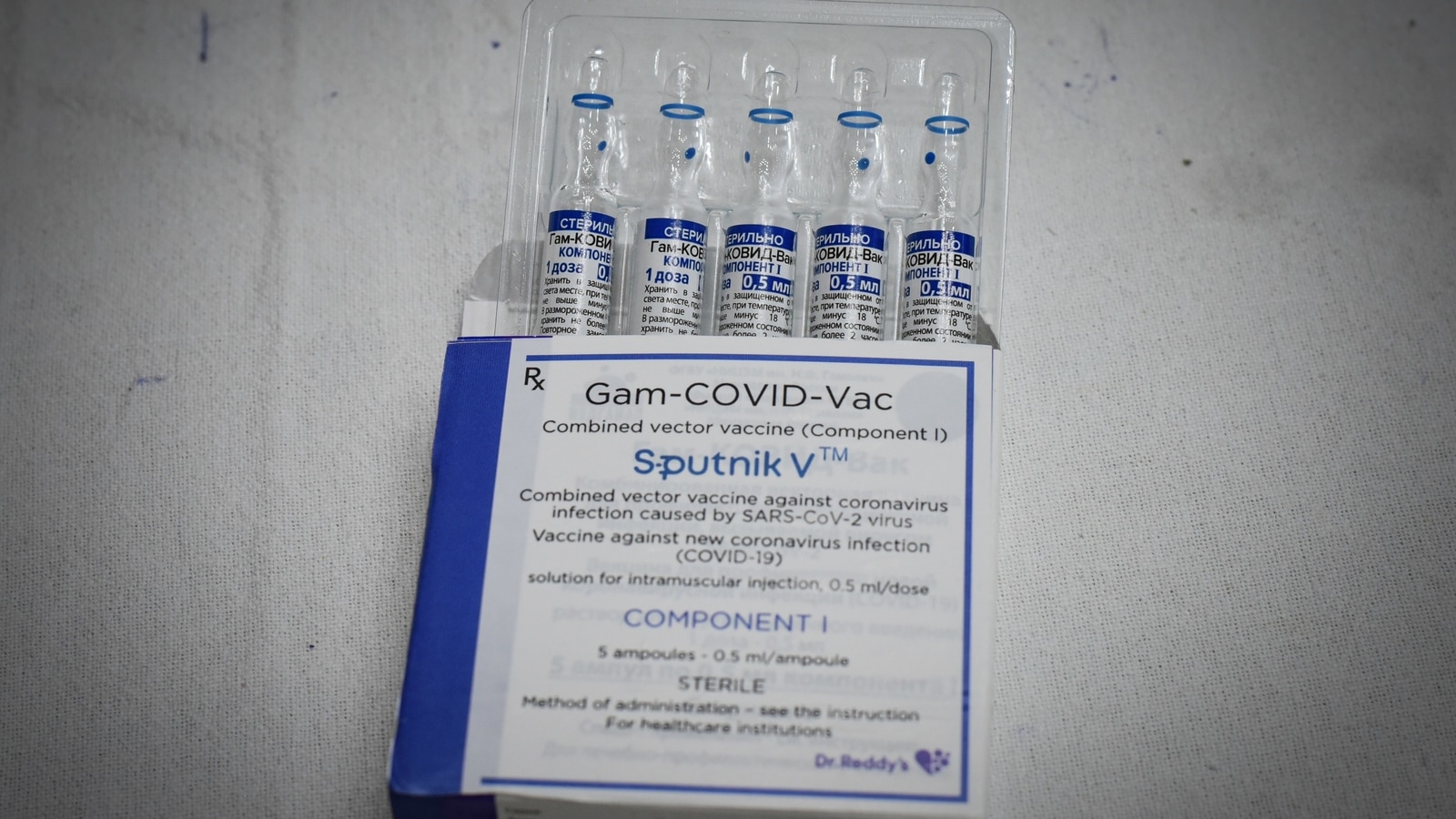
Kerala plans to sign agreement with RDIF to fill Sputnik V vaccine vials
Hindustan TimesGovernment funding work on four new COVID-19 vaccine candidates
The Hindu)
Serum Institute of India to start manufacturing Sputnik V in September, says RDIF
FirstpostSerum Institute to produce Russia’s Sputnik V vaccine
The Hindu
SII to produce 300 million doses of Sputnik V jabs annually
Live Mint
Serum Institute to start production of Sputnik V vaccine in India from September
India Today
Single dose of Sputnik V vaccine enough for recovered Covid-19 patients: Study
India Today
Sputnik V vaccine effective against Covid variants including Delta, claims study
India TodayDiscover Related


















)