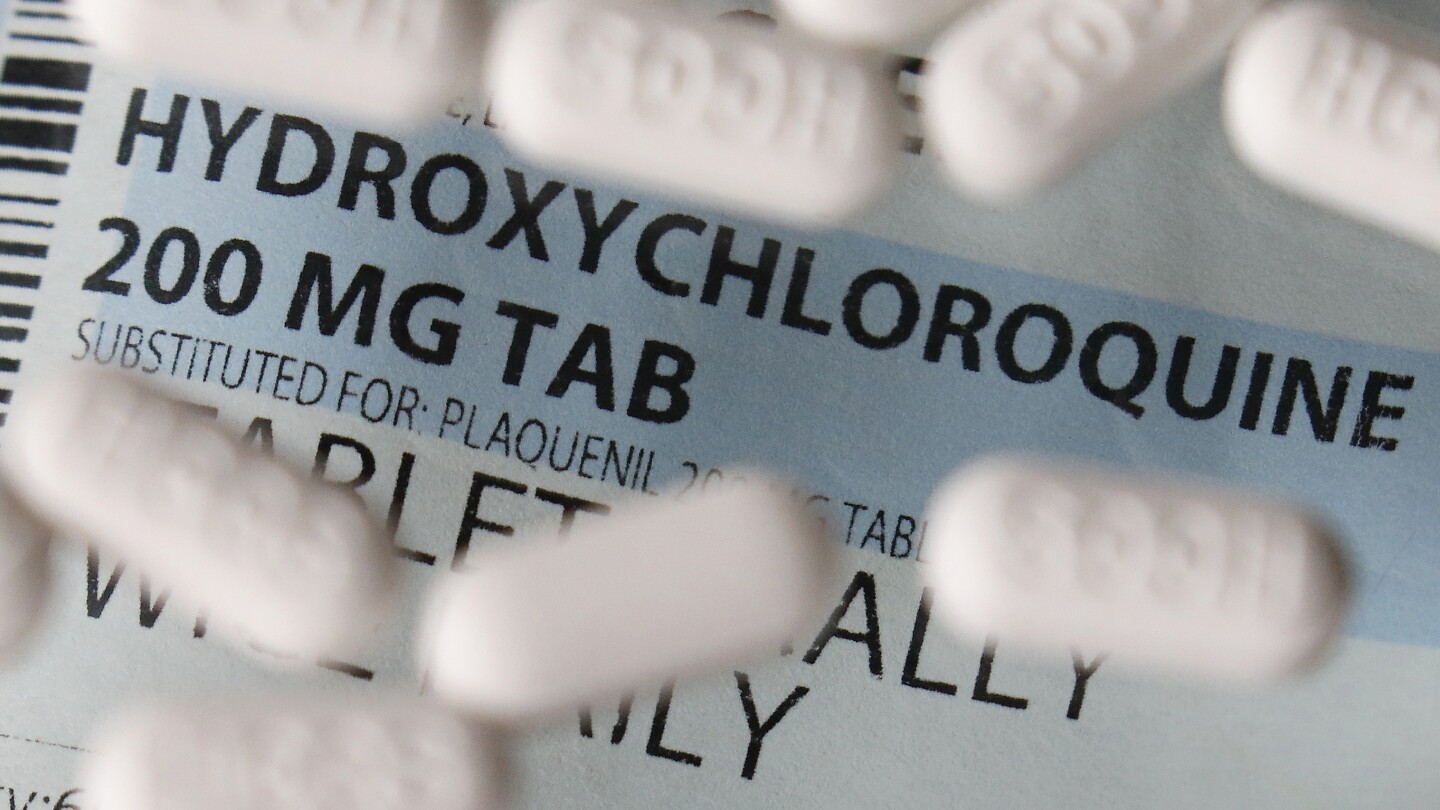
Posts mislead on Mayo Clinic’s hydroxychloroquine webpage
Associated PressCLAIM: The Mayo Clinic “quietly” updated its website in 2023 to say that hydroxychloroquine can now be used to treat COVID-19. A page about hydroxychloroquine on the hospital system’s website included outdated guidance that the malaria drug “may also be used to treat coronavirus in certain hospitalized patients.” But archives show the text has been there since at least May 2020, a month before the U.S. Food and Drug Administration revoked its emergency approval for using the drug to treat COVID. A screenshot in the post shows that that page read, in part: “Hydroxychloroquine may also be used to treat coronavirus in certain hospitalized patients.” However, archived versions of the website show there was nothing new about the language and it had been there since at least May 2020. The anti-malaria drug was authorized to be used for certain hospitalized COVID-19 patients, but the FDA revoked that authorization in June 2020, saying emerging data suggested it was “unlikely to be effective in treating COVID-19 for the authorized uses in the EUA.” In a statement on Monday, the Mayo Clinic said the organization was aware of the “inaccurate information” on its website, which it said had come from an outside vendor. The Mayo Clinic has now removed the hydroxychloroquine page, and the URL redirects to another page stating explicitly that it is not recommended as a treatment for COVID-19 Medical experts told the AP that the information on the page was consistent with the FDA’s guidance at the time it was written, but it has been clear since early 2020 that hydroxychloroquine treatments were not likely to help COVID-19 patients.
History of this topic

COVID treatments weren’t suppressed to OK vaccines’ emergency use
Associated Press
Panel: Trump staffers pushed unproven COVID treatment at FDA
Associated Press
What you should know about treating Covid-19
Live Mint
Remember Donald Trump-touted hydroxychloroquine? Study in India backs it as Covid-19 cure
India Today)
Do not use 'miracle cure' ivermectin on COVID-19 patients says WHO
Firstpost
Hydroxychloroquine shouldn’t be used as Covid preventive, says WHO
Hindustan TimesHydroxychloroquine push from backbenchers Craig Kelly and George Christensen a 'challenge' for Acting Chief Medical Officer Paul Kelly
ABC
Hydroxycloroquine does not help with Covid mortality; Improves ICU risk
NL Times)
Anti-malaria drug hydroxychloroquine ineffective against coronavirus, reveal UK RECOVERY Trials
Firstpost
Covid-19 vaccine developers prepare joint pledge on safety, standards
Live Mint
Amsterdam study shows hydroxychloroquine ineffective in Covid-19 treatment
NL Times
Everything you wanted to know about covid-19 treatments, in five charts
Live Mint
Where are we in finding a treatment for COVID-19?
Al JazeeraHydroxychloroquine is a poor coronavirus treatment but a perfect parable for our times
ABC
Hydroxychloroquine: Researchers publish scathing critique of Henry Ford study touted by the White House
CNN)
Trump Defends Use of Hydroxychloroquine, Says It Works in Early Stages of Covid-19 Infection
News 18
Hydroxychloroquine: Trump defends disproved Covid-19 treatment
Live Mint
Warnings against anti-malaria drug hydroxychloroquine to treat Covid-19 patients were politically motivated: Donald Trum
Live Mint
Video falsely touts hydroxychloroquine as COVID-19 cure
Associated Press
Twitter curbs Donald Trump Jr’s account over COVID-19 tweet
Live Mint)
Twitter Temporarily Limits Access to Donald Trump Jr's Account over False Hydroxychloroquine Claims
News 18
Trump again pushes unproven hydroxychloroquine as Covid-19 treatment
Live Mint
July 16 coronavirus news
CNN
Hydroxychloroquine doesn’t work in non-hospitalized patients
CNNNo room for complacency, says PM Modi after COVID-19 review
The Hindu
Trump's Claim That Hydroxychloroquine 'Doesn't Kill People' Looks Worse And Worse
Huff Post
US prescriptions for hydroxychloroquine skyrocketed from February to March, study finds
CNN
WHO ending hydroxychloroquine trial for COVID
LA Times
Hydroxychloroquine: NIH halts clinical trial after finding no additional benefit for Covid-19 patients
CNN
NIH Halts Hydroxychloroquine Study; Says 'Unlikely' To Help COVID-19 Patients
NPR
Hydroxychloroquine: US stockpile stuck with 63 million doses of the drug
CNN
Hydroxychloroquine: Brazil recommends unproven drug for pregnant women and children despite FDA ban
CNN
Steroid drug: A ‘breakthrough’ in the fight against coronavirus?
Al Jazeera
US revokes emergency use of drugs touted by Trump vs. virus
Associated PressFDA revokes emergency use authorisation for hydroxychloroquine
The Hindu
FDA Withdraws Emergency Use Authorization For Hydroxychloroquine
NPR
Hydroxychloroquine: FDA revokes authorization of drug Trump touted
CNNHydroxychloroquine does not reduce mortality, RECOVERY trial finds
The HinduCoronavirus | Avoid hydroxychloroquine in COVID-19 patients with severe disease, says Health Ministry protocol
The Hindu
Health Ministry okays HCQ for early course of Covid-19, remdesivir under emergency use
India Today
Govt reviews use of HCQ and antibiotic combo in treating coronavirus cases
Hindustan Times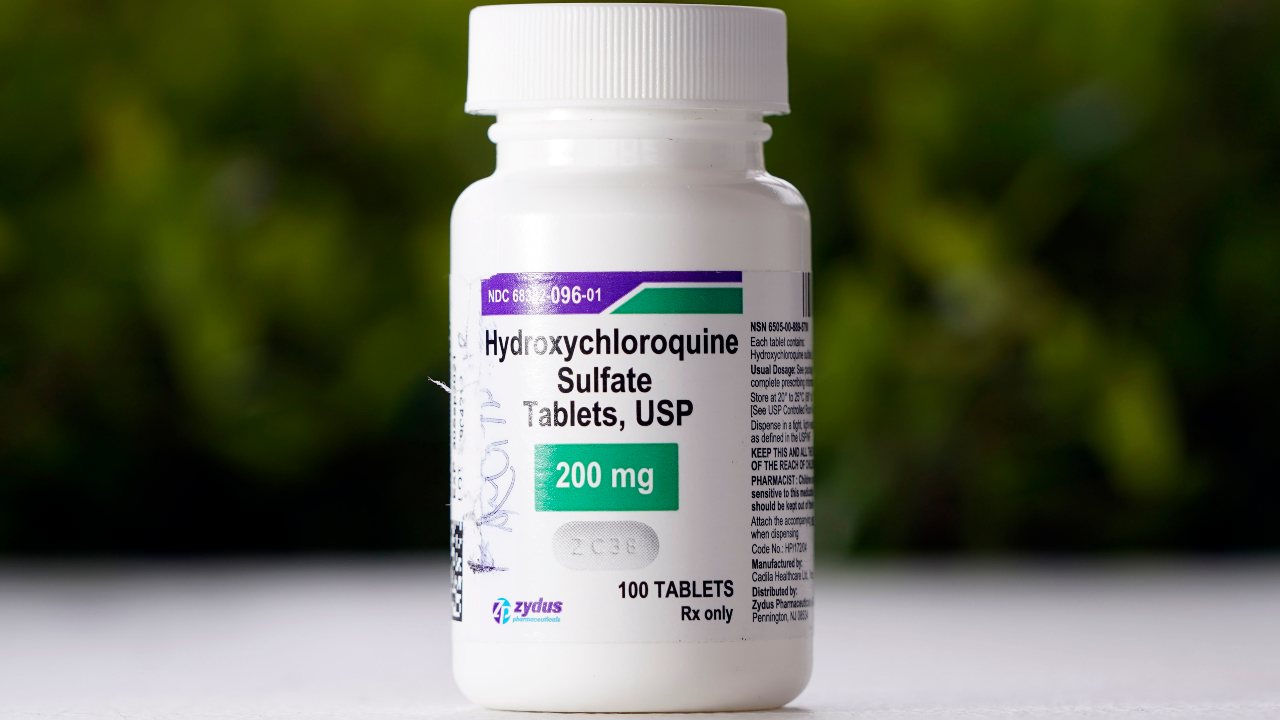)
The jury is still out on Hydroxychloroquine after the global debacle have left scientists uncertain of its usage
FirstpostDiscover Related





























)