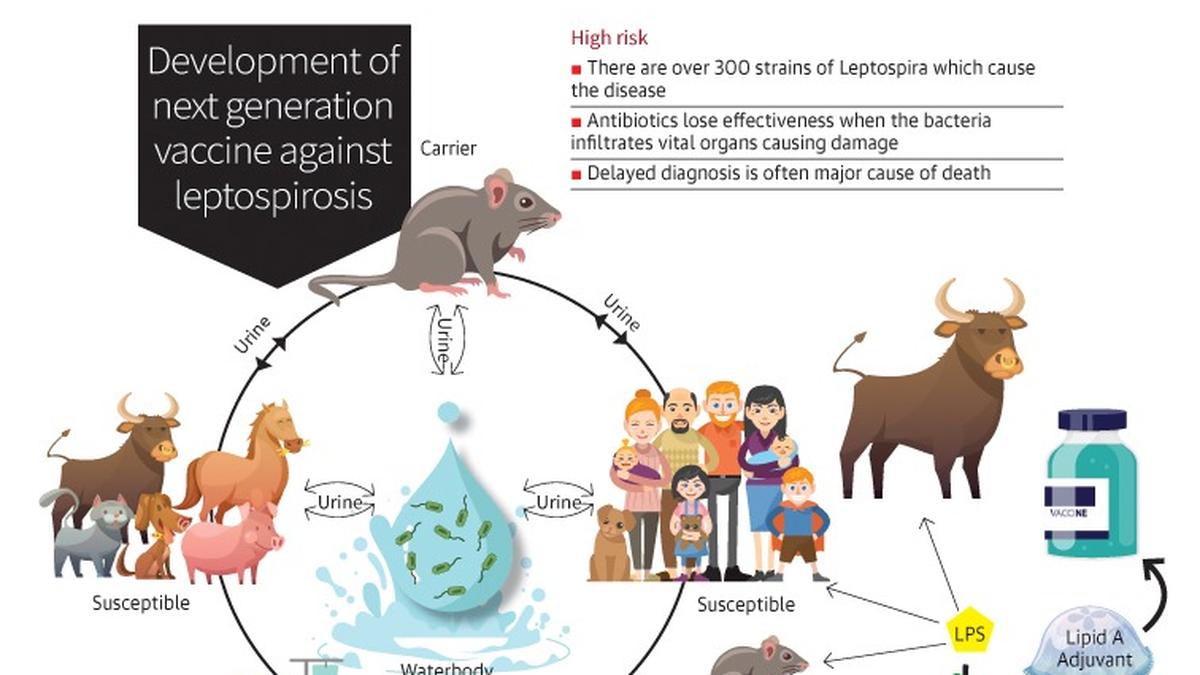
The vaccine race
The HinduON January 11, the genetic sequence of the virus causing the outbreak of the respiratory disease in Wuhan, China, was posted by a team at Fudan University in Shanghai on GenBank, a global online database maintained by the United States’ National Institutes of Health. Within three months, a period equivalent to travelling at the speed of light for vaccine development, the Oxford SARS-CoV-2 vaccine was in the first stage of human clinical trials. The speed was partly possible because Moderna, like the Oxford team, had been developing a vaccine for MERS and was able to switch the MERS spike protein with the SARS-CoV-2 virus protein. One of them, CanSino, a Tianjin-based company, like the Oxford team, used recombinant viral vector technology to create a vaccine which had its first human trials in mid March as well and is now undergoing phase 2 clinical trials. The U.S. government has invested $1.2 billion in the vaccine that Oxford University is developing, an amount that will pay for the remaining costs of developing the vaccine, manufacturing enough doses for clinical trials in the U.S., as well as at least part of the cost of “at risk manufacturing”, or beginning to manufacture hundreds of thousands of doses of a candidate vaccine before clinical trials are completed, so that if the trials are successful the vaccine will be available immediately to the public.
History of this topic

Phase-3 clinical trial of India's first dengue vaccine begins at KIMS-Bhubaneswar
New Indian Express
Recent breakthrough in COVID-19 vaccination: Indian Immunologicals Ltd unveils first needle-free booster vaccine
Hindustan Times
‘Safety is primary focus’: Covaxin-developer amid reports on AstraZeneca vaccine
Hindustan Times
With State prod, India can be world leader in vaccines
Hindustan Times
How India’s Vaccine Industry Will Save Millions of Children in Africa - News18
News 18
The Vaccine War: A tale of Indian scientists' triumph over COVID-19 amid the Left's propaganda to undermine indigenous vaccine
Op India
A new TB vaccine could save 8.5m lives over the next quarter of a century
The Economist
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
India restarts Covid vaccine production as new infections soar 30% in one day
The Independent
Old Covid Vaccines May Have Low Efficacy; India Should Update Covid Boosters, Dr Randeep Guleria Warns
News 18)
‘The Vial’ trailer out: Manoj Bajpayee narrates History TV18's documentary on India’s Covid-19 vaccine journey
Firstpost
Covid vaccine mission is a lesson for the world
Hindustan Times
Bharat Biotech starts dispatching nasal vaccines: Why it’s a big thing for India
Live Mint
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News
Over 7,000 Doses of Bharat Biotech's Intranasal Jab Ready for Market Rollout as Apex Lab Clears Batch 1
News 18
Nasal vaccine at govt centres in Delhi from Feb 15
Hindustan Times
India launches world’s 1st intranasal Covid vaccine: Check price, availability
Hindustan Times
What is nasal vaccine - iNCOVACC? All you need to know
India TV News
Centre rolls out first nasal Covid vaccine as booster
Hindustan Times
Health minister Mandaviya launches Bharat Biotech's nasal Covid vaccine
Hindustan Times
Private hospitals with advance orders to get intranasal COVID-19 shots soon
The Hindu
India’s first intra-nasal Covid-19 vaccine iNCOVACC launched
Live Mint
COVID-19: Mansukh Mandaviya launches world's 1st Made-in-India nasal vaccine iNCOVACC
India TV News
Bharat Biotech’s nasal vaccine likely to be launched on Jan 26
Hindustan Times
First Indian intranasal Covid vaccine by Bharat Biotech to be launched on Jan 26
India TV News
Braving a Viral Storm tells the tale of India’s COVID vaccine journey
The Hindu
Can Your Vaccine Protect You Against BF.7? India Isolates New Strain, Begins Study to Test Efficacy
News 18
Biological E, Bharat Biotech have stockpile of 250 million COVID vaccine doses
India TV News
Bharat Biotech’s intranasal covid vaccine priced at ₹325 for govt hospitals
Live Mint
Price of Bharat Biotech’s nasal Covid vaccine revealed. Details here
Live Mint
Bharat Biotech’s nasal vaccine on CoWIN app today: All you need to know
Live MintBharat Biotech’s nasal vaccine to be available as booster dose
The Hindu)
COVID-19: Centre approves Bharat Biotech's intranasal vaccine, to be available on CoWIN
Firstpost
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
World’s first intra-nasal vaccine for COVID gets CDSCO nod for restricted use
Live Mint
More vaccine choices: The Hindu Editorial on Bharat Biotech’s intranasal COVID-19 vaccine
The Hindu
Bharat Biotech's intranasal vaccine gets approval as heterologous booster
India Today
Bharat Biotech’s nasal covid vax gets CDSCO’s nod as heterologous booster
Live Mint
How the COVID-19 pandemic altered the vaccine story in India
The Hindu
AstraZeneca Nasal Spray Vaccine for Covid-19 Suffers Setback in Early Trial
News 18
Will Two ‘Made-in-India’ Vaccines Be the New Covid Boosters? News18 Decodes Differences
News 18
Bharat Biotech’s intra-nasal COVID vaccine gets emergency use approval
The Hindu
India's First Nasal Vaccine for Covid-19, Made by Bharat Biotech, Cleared for Emergency Use
News 18
Monkeypox vaccines: India puts emergency buying on hold for now - here’s why
Live Mint
India to launch first indigenous vaccine against cervical cancer on Sept 1
Hindustan Times
Tackling the new variants
Hindustan Times)
Bharat Biotech completes clinical development for phase III trials, booster doses for BBV154 intranasal COVID vaccine
Firstpost
COVID-19 intranasal vaccine Phase III trials over, proven safe, says Bharat Biotech
Deccan Chronicle
Monkeypox: 8 Indian firms express interest in developing vaccine
India TV News
Bharat Biotech expects regulator’s nod for intranasal COVID-19 vaccine this month
The HinduDiscover Related

















)