Potential COVID-19 vaccine by Zydus Cadila gets DCGI nod for human clinical trials
The HinduA potential COVID-19 vaccine indigenously developed by the Ahmedabad-based Zydus Cadila Healthcare Ltd. has got the nod from the Drugs Controller General of India for human clinical trials, according to a government source. Somani has given approval for the phase I and II clinical trials of the potential novel coronavirus vaccine developed by Zydus Cadila Healthcare Ltd. on Thursday after its animal studies were found to be successful,” an official source in the know of the developments told PTI. The assent for human trials was given after the company submitted data of clinical trial on animals to the DCGI, in which the vaccine candidate was found to be successful with respect to safety and immunogenicity, the source said. The country’s ‘first’ indigenous COVID-19 vaccine candidate Covaxin, developed by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research and National Institute of Virology, had recently got the nod for human clinical trials from the DCGI.
History of this topic

PopVax Announces that the U.S. National Institute of Allergy and Infectious Diseases will Conduct and Sponsor the U.S.-based Phase I Clinical Trial of PopVax’s Next-Generation mRNA-LNP COVID-19 Vaccin
Live Mint
Global Pandemic Threat: 100 Days to Save Lives
Hindustan Times
Recent breakthrough in COVID-19 vaccination: Indian Immunologicals Ltd unveils first needle-free booster vaccine
Hindustan Times
A new regime: On the Emergency Use Authorisation regime in India and clinical trials
The Hindu
Serum institute seeks to add Covovax on CoWIN portal as booster dose for adults
Hindustan Times
Covid vaccine mission is a lesson for the world
Hindustan Times
COVID-19: 3 lakh doses of intranasal vaccine iNCOVACC sent to hospitals, says Bharat Biotech's Krishna Ella
India TV News
India launches world’s 1st intranasal Covid vaccine: Check price, availability
Hindustan Times
What is nasal vaccine - iNCOVACC? All you need to know
India TV News
Health minister Mandaviya launches Bharat Biotech's nasal Covid vaccine
Hindustan Times
Private hospitals with advance orders to get intranasal COVID-19 shots soon
The Hindu
COVID-19: Mansukh Mandaviya launches world's 1st Made-in-India nasal vaccine iNCOVACC
India TV News
COVID-19: Health Minister Mansukh Mandaviya to launch world's 1st 'Made-in-India' nasal vaccine today
India TV News
Bharat Biotech’s nasal vaccine likely to be launched on Jan 26
Hindustan Times
First Indian intranasal Covid vaccine by Bharat Biotech to be launched on Jan 26
India TV News
DCGI Approves Market Authorisation for SII's Covid Vaccine Covovax as Heterologous Booster Dose
News 18
Braving a Viral Storm tells the tale of India’s COVID vaccine journey
The Hindu)
COVID-19: Covovax vaccine to get approval as booster dose in 10-15 days, says Poonawalla
Firstpost
No second Covid-19 booster dose required: Report
Live Mint
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The Hindu)
COVID-19: Centre approves Bharat Biotech's intranasal vaccine, to be available on CoWIN
Firstpost
SII Seeks Drug Regulator's Nod for Market Authorisation of Covovax Vaccine as Covid-19 Booster Dose
News 18India need not panic given excellent COVID-19 vaccination coverage: Adar Poonawalla
The Hindu
Bharat Biotech urges Centre to include its intranasal COVID vaccine in CoWIN portal
Deccan Chronicle
World’s first intra-nasal vaccine for COVID gets CDSCO nod for restricted use
Live Mint
CDSCO nod for Bharat Biotech’s COVID-19 intranasal vaccine under restricted use
The Hindu
Health Ministry not to procure fresh COVID-19 vaccines; surrenders ₹4,237 crore from vaccination budget
The Hindu
How the COVID-19 pandemic altered the vaccine story in India
The Hindu
AstraZeneca Nasal Spray Vaccine for Covid-19 Suffers Setback in Early Trial
News 18
Will Two ‘Made-in-India’ Vaccines Be the New Covid Boosters? News18 Decodes Differences
News 18
Bharat Biotech’s intra-nasal COVID vaccine gets emergency use approval
The Hindu
India's First Nasal Vaccine for Covid-19, Made by Bharat Biotech, Cleared for Emergency Use
News 18
Covid-19: India's first intranasal vaccine by Bharat Biotech gets DCGI approval
India TV News
China Approves World's First Inhalable Covid-19 Vaccine
News 18
COVID-19 intranasal vaccine Phase III trials over, proven safe, says Bharat Biotech
Deccan Chronicle
COVID-19: Corbevax to be given as boosters at vaccination centres from today
Live Mint
Corbevax Likely to be Available As Covid Booster Dose at Vaccination Centres from Friday
News 18
Corbevax as Covid booster for adults from August 12. Check details
India Today
Corbevax approved as booster for those jabbed with Covishield, Covaxin: Report
Live Mint
In A First, Biological E's Corbevax Approved As Covid Booster for Covishield, Covaxin Recipients
News 18
Govt Nod for Corbevax as Booster for Adults Vaccinated with Covishield, Covaxin Soon
News 18
Adults may get different vaccine as booster shot instead of Covishield, Covaxin
Live Mint
Bharat Biotech expects regulator’s nod for intranasal COVID-19 vaccine this month
The Hindu
Free COVID-19 booster shots for all adults for 75 days from July 15 at government centres
The Hindu
Serum Institute gets govt nod to export 32.4 lakh doses of Covovax vaccine to US
India Today
Gennova Biopharmaceuticals' mRNA vaccine gets DCGI approval for emergency use
India TV News
Allowing Corbevax as booster for those vaccinated with Coivishield, Covaxin likely to be considered by NTAGI
The Hindu
Over 42 lakh deaths in India prevented by COVID-19 vaccines in 2021: Lancet study
The Hindu
Bharat Biotech Nasal Covid Vaccine's Phase 3 Trials Completed
News 18)
COVID-19: Nasal vaccine's phase 3 trial completed, informs Bharat Biotech chief
FirstpostDiscover Related



















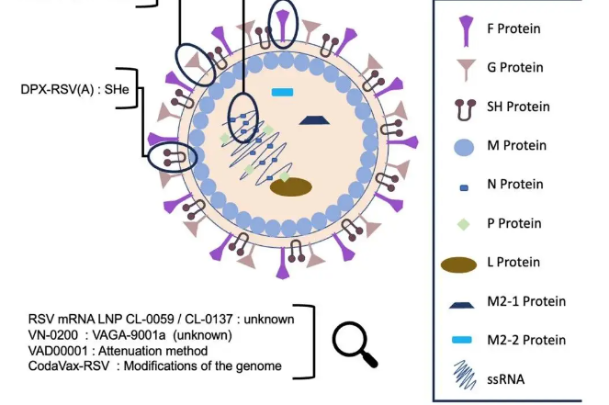



)