)
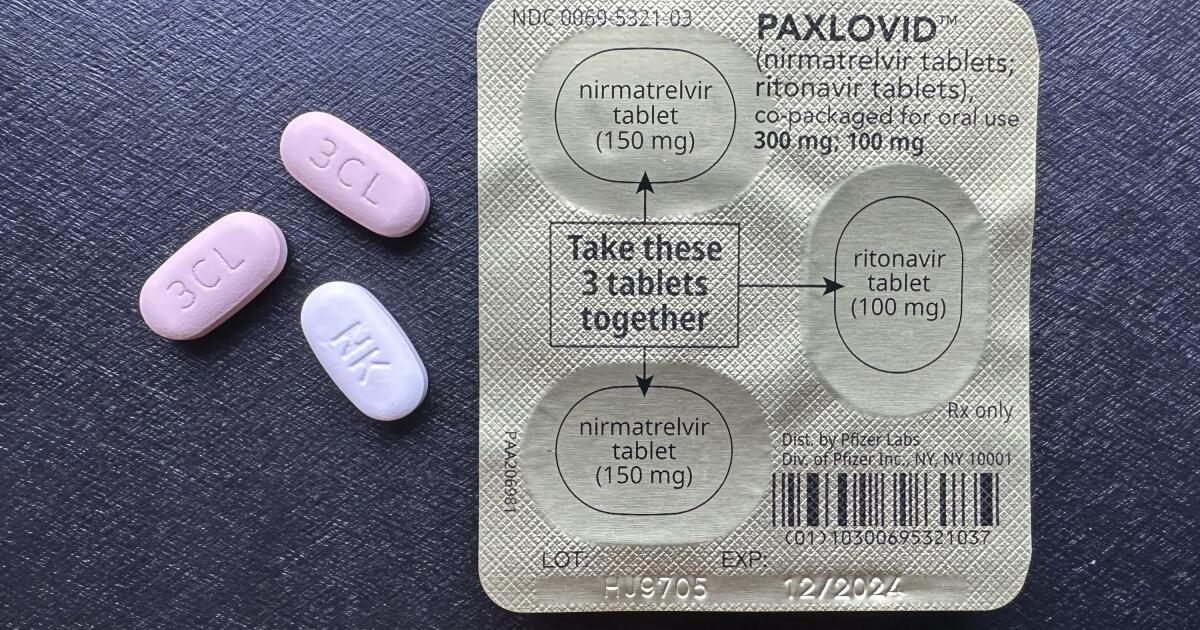
Kamala Harris' COVID-19 being treated by Paxlovid: All you need to know about Pfizer's oral drug
FirstpostPaxlovid, the oral drug, reduces the risk of COVID-19-associated hospitalisation or death by 89 per cent in those who receive treatment within three days of symptom onset The fight against coronavirus received yet another boost last Friday when the World Health Organization “strongly recommended” Pfizer’s COVID-19 antiviral pill Paxlovid for patients with milder forms of the disease who were still at a high risk of hospitalisation. The WHO’s experts said in the BMJ medical journal that the United States’ pharma giant Pfizer’s combination of nirmatrelvir and ritonavir was the “superior choice” of treatment for unvaccinated, elderly or immunocompromised people with COVID-19. An interim analysis showed that Paxlovid reduced the risk of COVID-19-associated hospitalisation or death by 89 per cent in those who received treatment within three days of symptom onset. Paxlovid in India In March, the Medicines Patent Pool had signed a licence agreement with 35 generic drug makers to manufacture the generic version of Pfizer’s oral COVID-19 treatment.
History of this topic
COVID-19 treatments to enter the market with a hefty price tag
Associated Press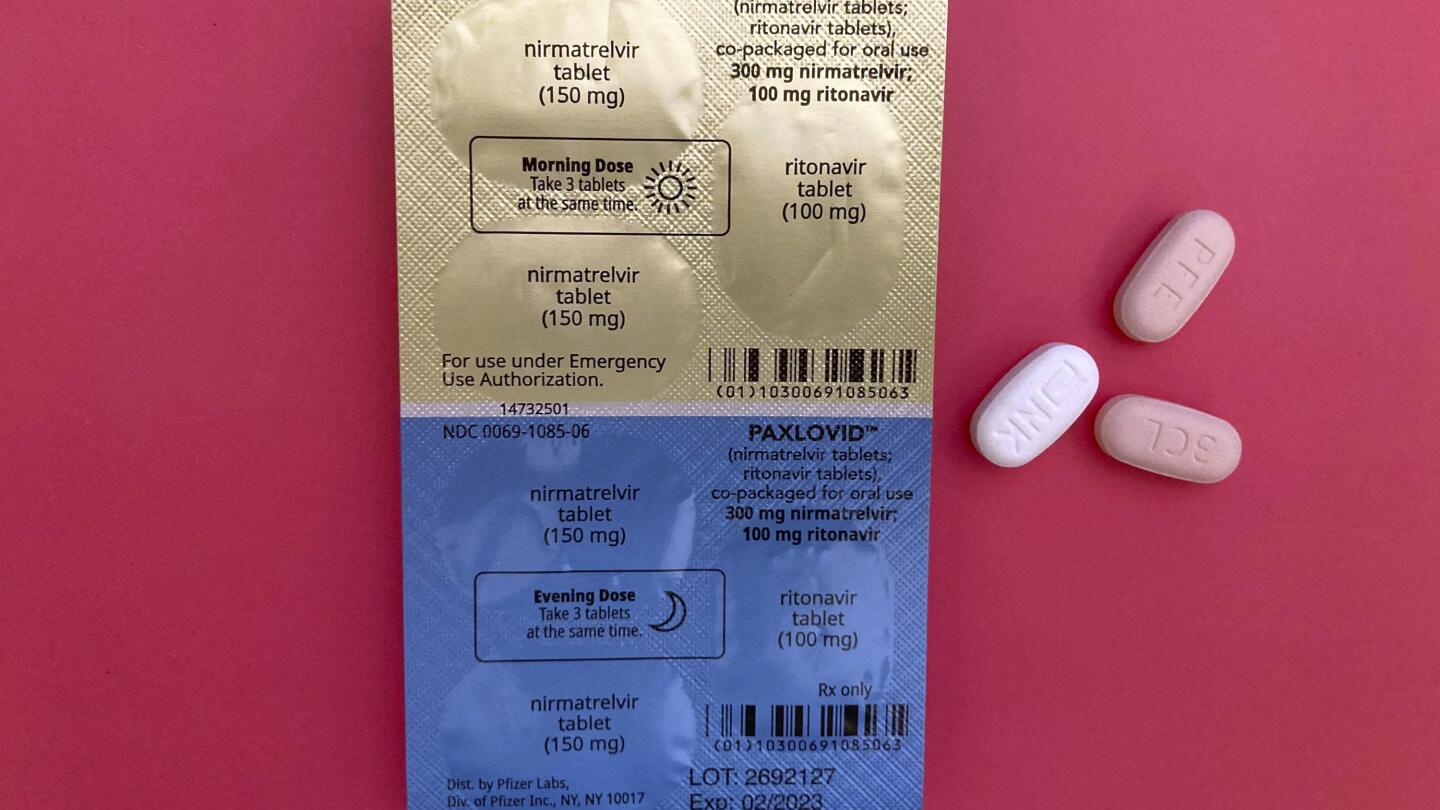
COVID pill Paxlovid gets full FDA approval after more than a year of emergency use
Associated Press
Pfizer’s New Oral Anti-Viral Drug Lowers Risk of Post-Covid Condition, Study Shows
News 18)
COVID-19 pill Paxlovid moves closer to full FDA approval
Firstpost
COVID-19 pill Paxlovid moves closer to full FDA approval
Associated Press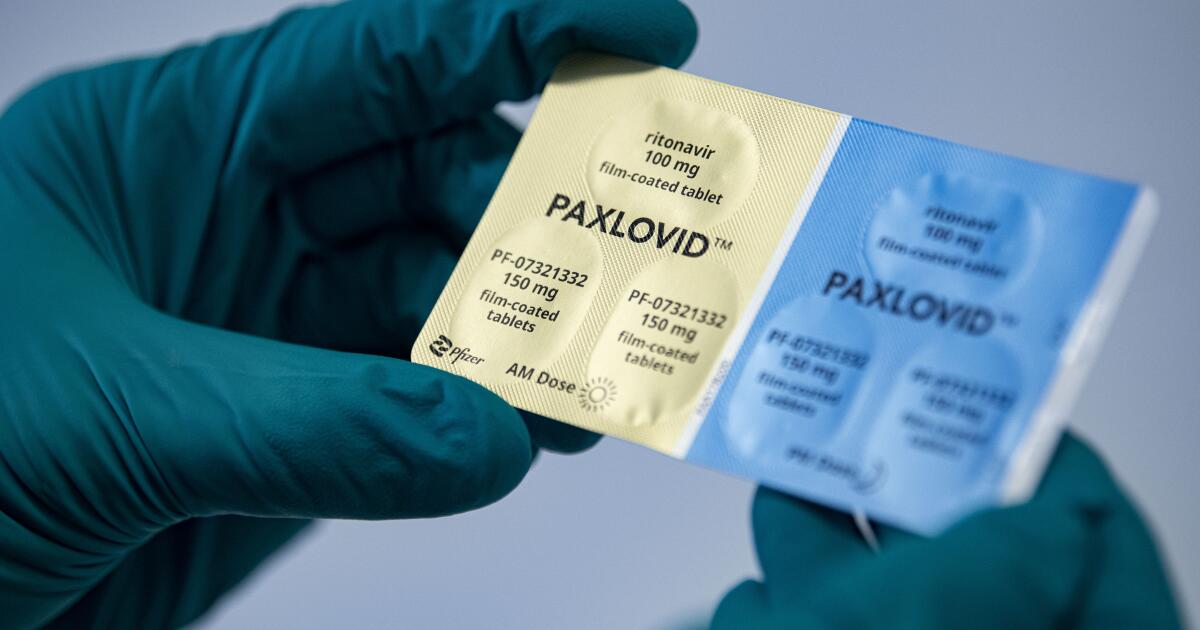
COVID-19 pill Paxlovid moves closer to full FDA approval
LA Times‘Fake’ Indian COVID-19 medicines flood China’s black market amid surge in cases
The Hindu
Chinese turning to Indian drugs on black market amid Covid spike
India Today
Staying prepared: On scaling up the pace of COVID-19 genome sequencing
The Hindu)
Amid COVID surge, China approves America’s Paxlovid, an anti-viral drug: Why this is an unlikely move
Firstpost
Hetero emerges first to get WHO Prequalification for Covid drug Nirmacom
Deccan Chronicle
Antiviral Covid treatment now available in the Netherlands for €1,250
NL Times
Pfizer CEO tests positive for COVID-19, has mild symptoms
LA TimesOral pill for COVID-19 approved by regulator
China Daily
Zenara Pharma gets CDSCO nod to make copy of Pfizer’s Paxlovid
The Hindu
US allows pharmacists to prescribe Pfizer’s COVID-19 pill
Associated Press
FDA allows pharmacists to prescribe Pfizer’s Paxlovid COVID pill
LA Times
Pfizer to offer low-cost medicines, vaccines to poor nations
Associated Press
Rare Cases Of COVID Returning Pose Questions For Pfizer Pill
Huff Post
White House expands access to life-saving COVID-19 drug Paxlovid
Salon
Why Pfizer's new drug Paxlovid raises hopes against Covid
India Today
Administration expands availability of COVID antiviral pill
Associated Press
Biden admin to promote availability of COVID antiviral pill
Associated Press
WHO recommends Pfizer's pill Paxlovid for mild Covid cases
India Today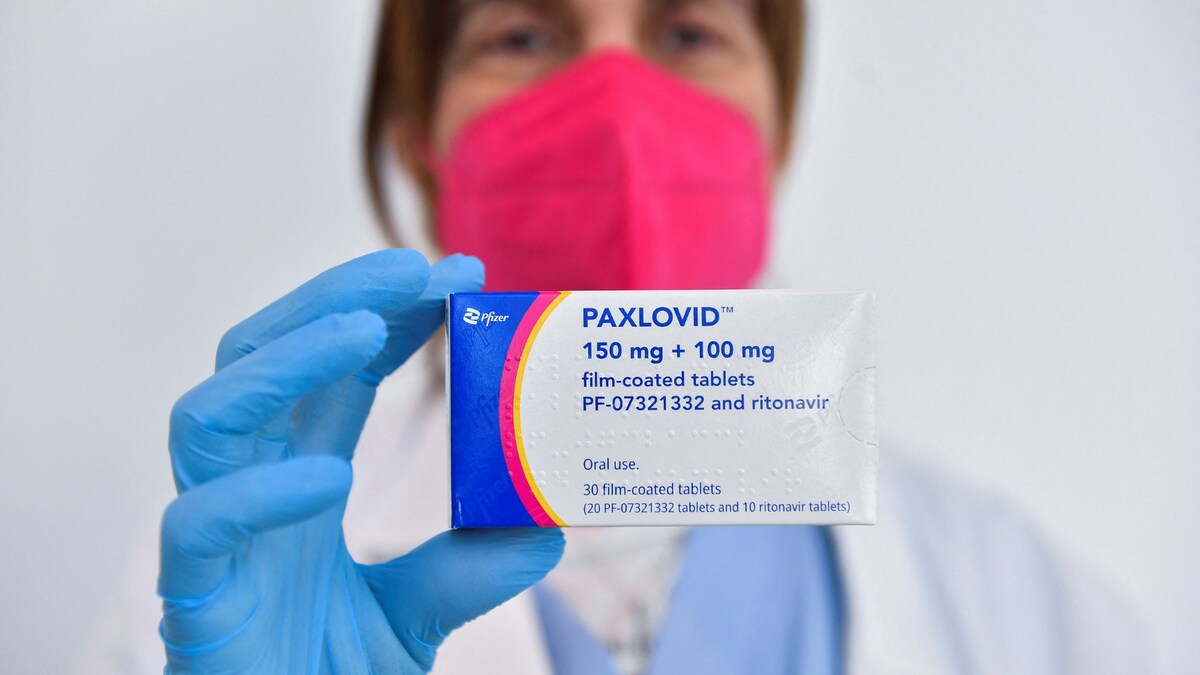
What is Paxlovid and Who Can Take Pfizer’s Covid-19 Wonder Drug ‘Strongly Recommended’ by WHO?
News 18
WHO 'strongly recommends' Pfizer's Covid pill | 5 things to know about Paxlovid
Hindustan Times
Covid-19 pill competing with Pfizer’s looks for quick approval in Japan
Live Mint
Opinion | Covid cases spiralling: Exercise utmost caution
India TV News
Pfizer’s Covid-19 antiviral pill was hailed as a game-changer, but supplies are scarce
CNN)
Optimus Pharma to launch anti-COVID pill Molnupiravir next week; all you need to know
Firstpost
2 more Covid vaccines Covovax and Corbevax, and anti-viral drug Molnupiravir cleared for use in India
India TV News
A shot in the arm: The Hindu Editorial on new modes of COVID-19 treatment
The Hindu
Chandrakant Lahariya | FDA approval of Covid pill a sign of hope for the world
Deccan Chronicle
Pfizer's oral Covid-19 pill gets US authorisation for at-home use
India TV News
Pfizer Pill Becomes First US-authorized Home Covid-19 Treatment
News 18
Pfizer pill becomes 1st U.S.-authorized home COVID treatment
LA Times
European Medicines Agency recommends emergency use of Pfizer Covid-19 pill
India TV News
As Omicron peaks, here's the status of 3 Covid-19 drugs Molnupiravir, Paxlovid and Ronapreve
India Today
Pfizer Covid-19 antiviral pill: Biden says he’s ‘encouraged by the promising data’
CNN
Merck’s covid-19 pill backed by FDA advisers
Live Mint
Merck’s covid-19 pill to be reviewed by FDA advisers
Live Mint
Finally, pills to treat Covid-19! All you need to know about Pfizer's Paxlovid and Merck's Molnupiravir
India Today
MSD in talks to give additional licences for molnupiravir
Live Mint
Pfizer in final stages to get nod for covid vaccine in India: CEO Albert Bourla
Live Mint
Pfizer's Covid vaccine in final stages of getting approval in India: CEO Albert Bourla
India Today
Told India no safety concern with its Covid-19 vaccine: Pfizer
India Today
Discussions on with Indian govt for expedited approval of COVID-19 vaccine: Pfizer
India TV NewsCoronavirus | Pfizer seeks emergency use authorisation for its COVID-19 vaccine in India
The Hindu)
Pfizer seeks emergency use authorisation of its COVID-19 vaccine in India
FirstpostDiscover Related