Explained | Molnupiravir, Merck’s new drug to treat COVID-19
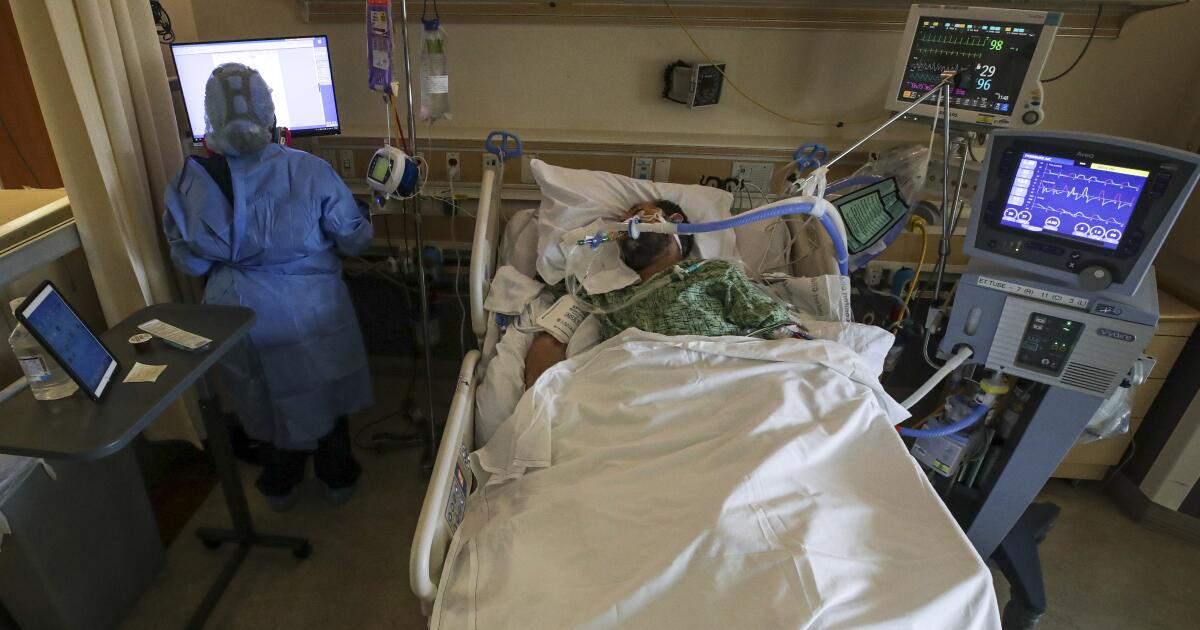
The HinduWhy is there much excitement about molnupiravir, the investigational new drug for COVID-19? Pharmaceutical major Merck and Ridgeback Biotherapeutics announced via a press release on October 1 the early results from Phase-3 trials that its anti-viral drug molnupiravir halved the chances of hospitalisation in COVID-19 patients with mild or moderate disease. Several noted clinicians have said that these are promising results, and what is particularly encouraging is that molnupiravir is a pill, unlike other drugs — with similar efficacy — used in COVID-19 treatment, which needs to be administered intravenously. The drug has so far been tested only in patients with mild-to-moderate COVID-19, had started treatment within five days of testing positive and had at least one risk factor that increased their risk for severe disease. Another positive factor is that recruitment for the phase-3 trial, that originally envisaged recruiting 1,500 patients for testing the drug’s potency, was halted early by an independent data monitoring committee because the data appeared so encouraging that it would be unethical to delay making the drug more widely available.
History of this topic

Molnupiravir Leads To Quicker Recovery In Vaccinated Adults Infected With Covid Does Not Reduce Hospital Admission Or Death Study In Lancet
ABP NewsVINCOV-19, country’s first anti-dote for COVID, ready for phase 3 trials, market authorisation
The Hindu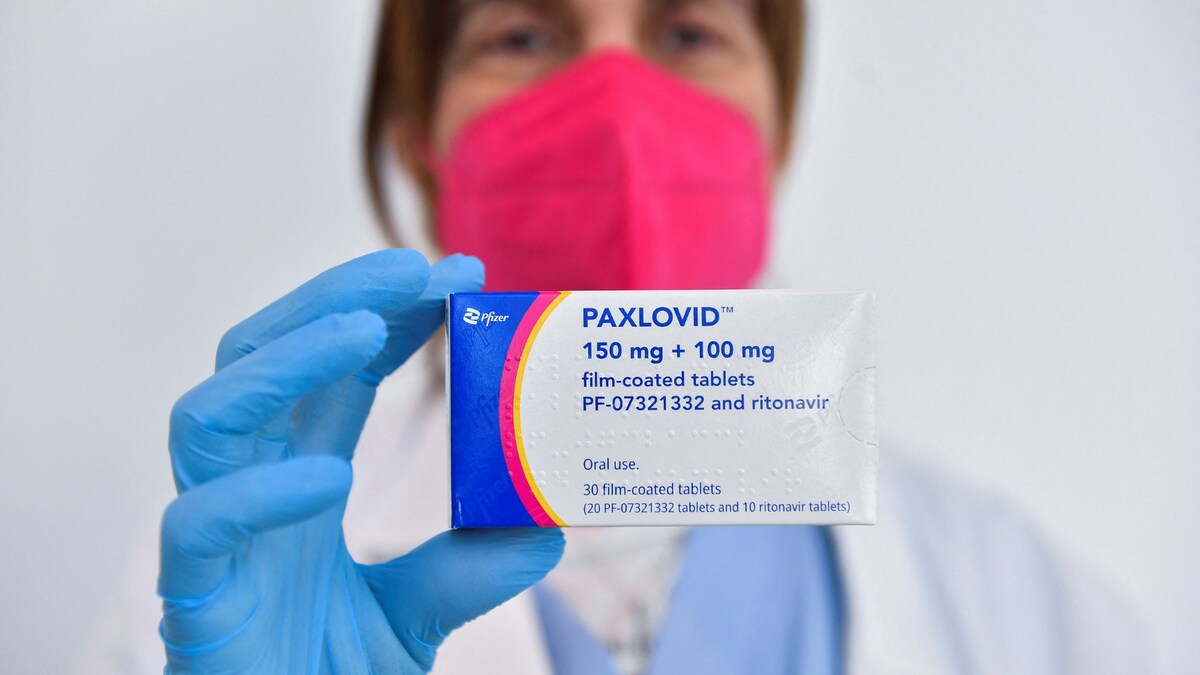
What is Paxlovid and Who Can Take Pfizer’s Covid-19 Wonder Drug ‘Strongly Recommended’ by WHO?
News 18
Health Matters: Dear Drug Regulator, Silence is Not Always Golden, Esp on WHO Pausing Covaxin Supply
News 18
COVID-19 pills could be a game-changer, but doctors trying to use them face hurdles
LA Times)
'Prepare for new COVID-19 variants': Joe Biden tells pandemic-weary Americans
Firstpost
Covid-19 pill competing with Pfizer’s looks for quick approval in Japan
Live Mint
Generic Drugmakers to Make Cheap Versions of Merck's Covid-19 Pill Molnupiravir for Poorer Nations
News 18
Cheap version of Merck Covid pill to be made for poorer nations
India TodayMolnupiravir kept out of revised clinical guidelines for management of adult COVID-19 patients
The Hindu
WHO adds new drugs to COVID treatments amid Omicron surge
Al JazeeraNo molnupiravir: ICMR team
The Hindu)
Lupin launches antiviral medication Molnulup for COVID-19 treatment in India
FirstpostCovid antiviral drug Molnupiravir has major safety concerns: ICMR chief
The Hindu
Molnupiravir, oral anti-Covid medicine, set for launch in India; to cost Rs 649 per strip
India TV News
EXPLAINED: As Covid Antiviral Molnupiravir Launches In India, Here's All You Need To Know About The Pill
News 18
Mankind Pharma to Launch Cheapest Covid Antiviral Drug Molnupiravir at Rs 35 Per Capsule by Next Week
News 18
New Covid-19 pills carry risks: What patients should know
Live Mint)
Optimus Pharma to launch anti-COVID pill Molnupiravir next week; all you need to know
Firstpost
Approved by India, Full Treatment of Anti-Viral Drug Molnupiravir May Cost Up to Rs 3,000: Report
News 18
Molnupiravir Anti-Viral Pill to be Tested on 13,000 More Patients, Makers to Submit Report in 3 Months
News 18Paxlovid and Molnupiravir | A new front in the battle against the pandemic
The Hindu
A shot in the arm: The Hindu Editorial on new modes of COVID-19 treatment
The Hindu
Live updates: Japan approves use of Merck's COVID-19 pill
The Independent
Chandrakant Lahariya | FDA approval of Covid pill a sign of hope for the world
Deccan Chronicle
US adds Merck pill as 2nd easy-to-use drug against COVID-19
Associated Press
US adds Merck pill as 2nd easy-to-use drug against Covid-19
India Today
U.S. adds Merck pill as 2nd easy-to-use drug against COVID-19
LA Times
Merck’s at-home antiviral COVID-19 pill approved by US regulator
Al Jazeera
France scraps order for Merck's Covid antiviral drug after disappointing trial results
India Today
As Omicron peaks, here's the status of 3 Covid-19 drugs Molnupiravir, Paxlovid and Ronapreve
India Today
Merck's Covid-19 pill Molnupiravir found to reduce hospitalisation, death risks
India Today
Pfizer confirms COVID pill’s results, potency versus omicron
Associated PressPfizer confirms COVID pill’s results, potency versus Omicron
The Hindu
Pfizer says its COVID-19 treatment pill works against the Omicron variant
LA Times
UK planning to rollout Merck's anti-Covid pill as soon as Christmas: Report
Hindustan Times
Merck’s covid-19 pill backed by FDA advisers
Live Mint
US panel backs first-of-a-kind COVID-19 pill from Merck
Associated Press
US panel backs Merck's first-of-a-kind Covid-19 pill
Hindustan Times
US health panel endorses Merck’s COVID-19 pill
Al Jazeera
Merck’s covid-19 pill to be reviewed by FDA advisers
Live Mint
New antiviral drugs are coming for COVID. Here's what you need to know
NPR
Merck’s COVID pill shows lower efficacy in updated study
Al Jazeera
Merck asks EU drug regulator to authorise its Covid-19 pill
Hindustan Times
EU regulator recommends Merck’s COVID-19 pill for emergency use
Al Jazeera
Finally, pills to treat Covid-19! All you need to know about Pfizer's Paxlovid and Merck's Molnupiravir
India Today
Pfizer says COVID-19 pill cut hospital, death risk by 90%
Associated Press
Pfizer says Covid-19 pill is 89% effective in preliminary assessment
Live Mint
Pfizer says its COVID-19 pill cuts hospitalisations, death by 89%
Al Jazeera
Britain becomes first country to approve Merck's Covid-19 pill molnupiravir
India TodayDiscover Related