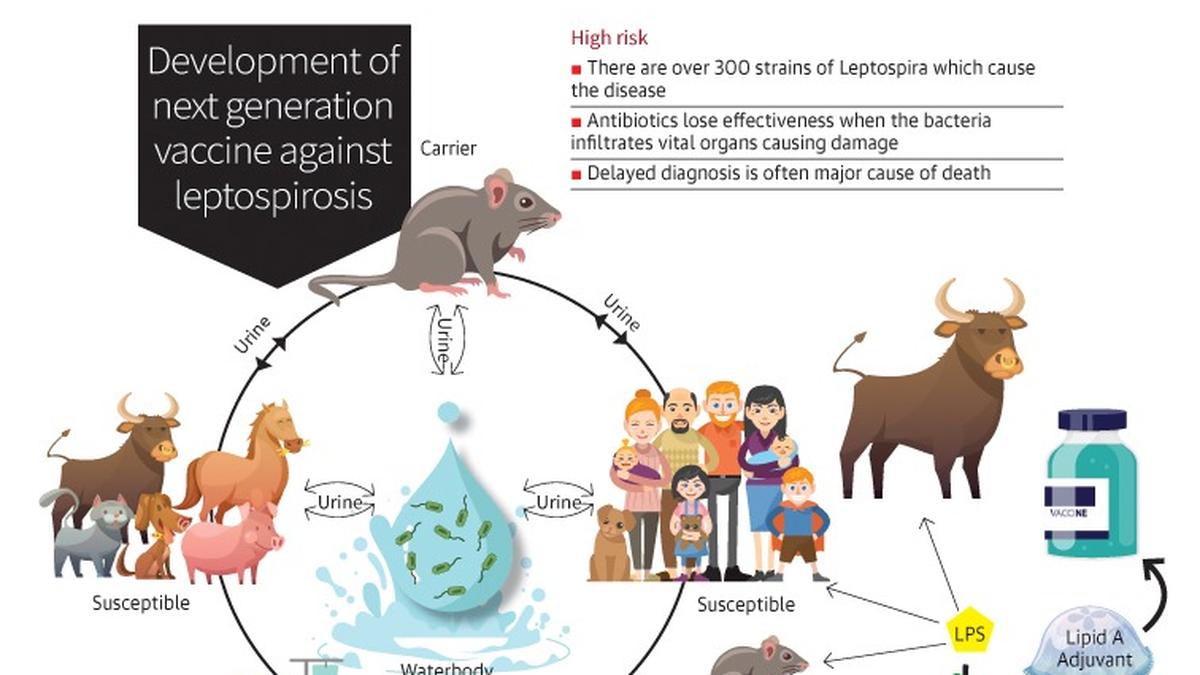
Vaccine worries
The HinduON January 15, the production licences of three vaccine-manufacturing public enterprises under the Ministry of Health and Family Welfare were cancelled, and they were ordered to suspend production forthwith by the then Drugs Controller General of India, M. Venkateswarlu, for reasons of non-compliance with good manufacturing practice norms under the Indian Drugs and Cosmetics Act of 1945. A centralised vaccine park, which will have state-of-the-art infrastructure for production and research for both UIP and non-UIP vaccines, including the new generation vaccines, is proposed to be set up in Chengalpattu near Chennaii in lieu of these three units. In fact, some people fear that the vaccine park move may just be a ruse to shut down these public sector units. While the government did not have second thoughts about importing a non-WHO pre-qualified vaccine to save lives, it has decided to shut down units producing life-saving vaccines whose quality is not in doubt for the same reason, namely, that their products are not WHO-GMP certified. Justifying the move to have a vaccine park at Chengalpattu and have HLL set it up, he said: There is no space at Kasauli or Coonoor even to build new facilities there.
History of this topic
Discover Related